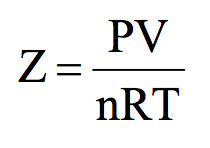20.If Z is a compressibility factor, van der Waals equation at low
4.9 (294) · $ 8.99 · In stock
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

Real Gases and the van der Waals Equation Explained

Solved We begin by showing that the compressibility factor

physical chemistry - Is the compressibility factor smaller or

Deviations from ideal gas behaviour, intermolecular forces, Van

Solved 2. (20 points) At low pressures, the compressibility
The compressibility factor for one mol of a vanderwalls gas at 0

If Z is a compressibility factor, van der Waals' equation at low

The given graph represent the variations of Z Compressibility

Van der Waals equation - Wikipedia

16.4: The Law of Corresponding States - Chemistry LibreTexts

At a high pressure, the compressibility factor (Z) of a real gas is us

66. If z is the compressibility factor, van der Waals equation low

66. If z is the compressibility factor, van der Waals equation low