Straightforward Access to Terminally Disubstituted Electron‐Deficient Alkylidene Cyclopent‐2‐en‐4‐ones through Olefination with α‐Carbonyl and α‐Cyano Secondary Alkyl Sulfones - Trifonov - 2021 - European Journal of Organic Chemistry - Wiley Online Library
5 (169) · $ 20.50 · In stock

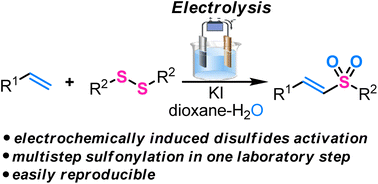
Disulfides as versatile starting reagents: effective sulfonylation of alkenes with disulfides under electrochemical conditions - Organic Chemistry Frontiers (RSC Publishing)

Catalysts, Free Full-Text

Yb(OTf)3-catalyzed cyclization of an N-silylenamine with 2-methylene-1,3-cyclohexanedione to afford a 7,8-dihydroquinolin-5(6H)-one derivative and its application to the one-pot conversion to a 2,3,5-trisubstituted quinoline derivative - ScienceDirect
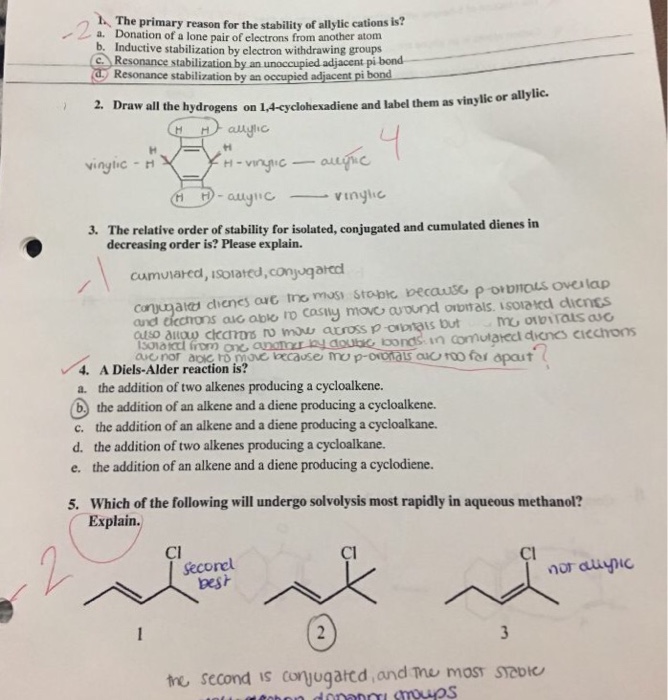
Solved The primary reason for the stability of allylic
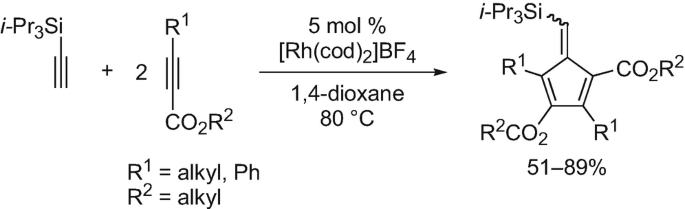
Synthesis and Application of Highly Substituted Cyclopentadienes
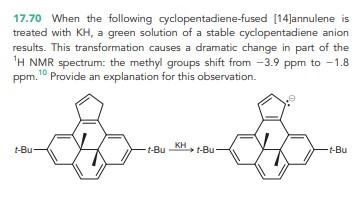
Solved 17.70 When the following cyclopentadiene-fused

Visible-light-mediated interrupted Cloke-Wilson rearrangement of cyclopropyl ketones to construct oxy-bridged macrocyclic framework - ScienceDirect

Chapter 14 - Orbital Interactions 2: Extended Pi Systems, Conjugation, and Aromaticity Flashcards
![2 + 2] Cycloaddition of phosphaalkenes as a key step for the reductive coupling of diaryl ketones to tetraaryl olefins - Chemical Science (RSC Publishing) DOI:10.1039/D2SC03073J](https://pubs.rsc.org/image/article/2022/SC/d2sc03073j/d2sc03073j-f1_hi-res.gif)
2 + 2] Cycloaddition of phosphaalkenes as a key step for the reductive coupling of diaryl ketones to tetraaryl olefins - Chemical Science (RSC Publishing) DOI:10.1039/D2SC03073J

Broadening the scope of carbonyl olefination reactions using a new electrophotocatalytic approach
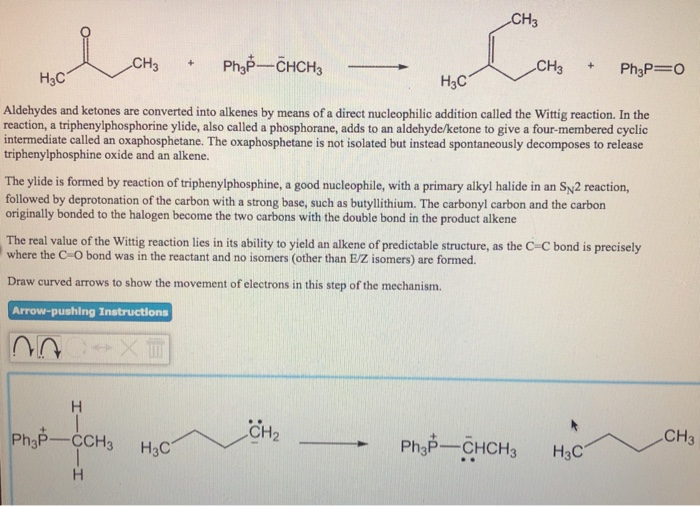
Solved This question has parts to it. I will rate. please






![Metal‐, Photocatalyst‐, Light‐ and Electrochemical‐Free C‐3 Trifluoromethylation of Quinoxalin‐2(1H)‐ones, Imidazo[1,2‐a]pyridines and 2H‐Indazoles - Dutta - 2021 - ChemistrySelect - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/fa54386e-10f5-492b-8024-773ca1618fe6/slct202004631-toc-0001-m.png)
