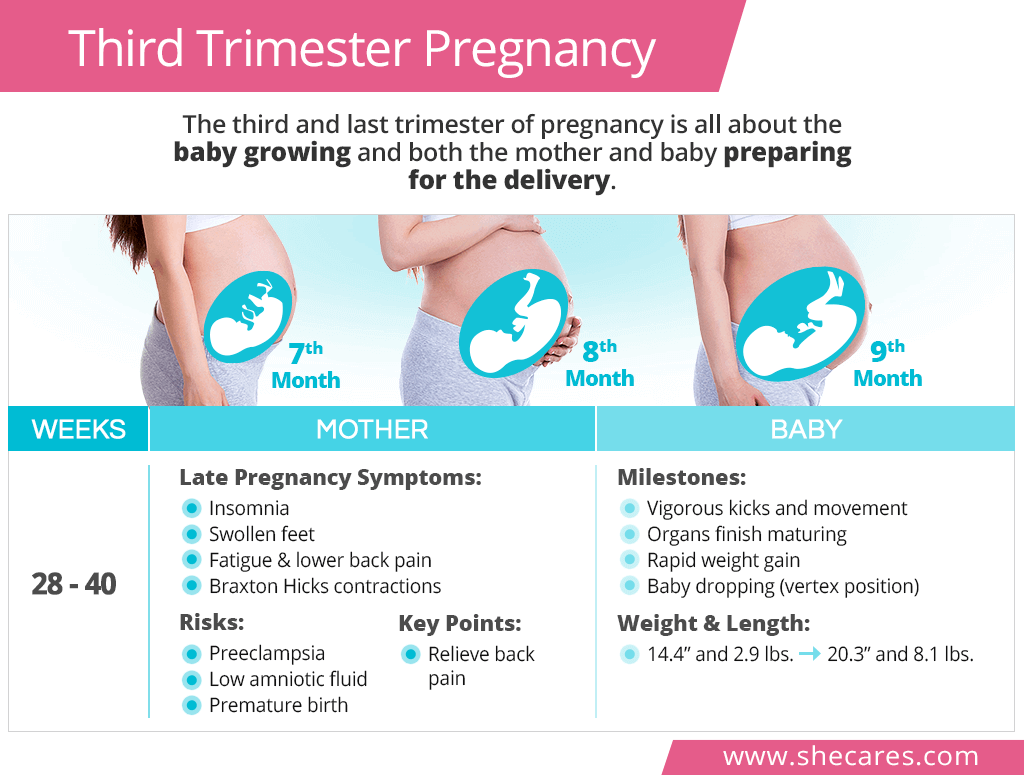
Applications for Medical Device Investigational Testing Authorizations Guidance Document
4.5 (250) · $ 10.99 · In stock

Applications for Medical Device Investigational Testing Authorizations Guidance Document

Class II - IV Medical Device Investigational Testing in Canada - Vantage BioTrials
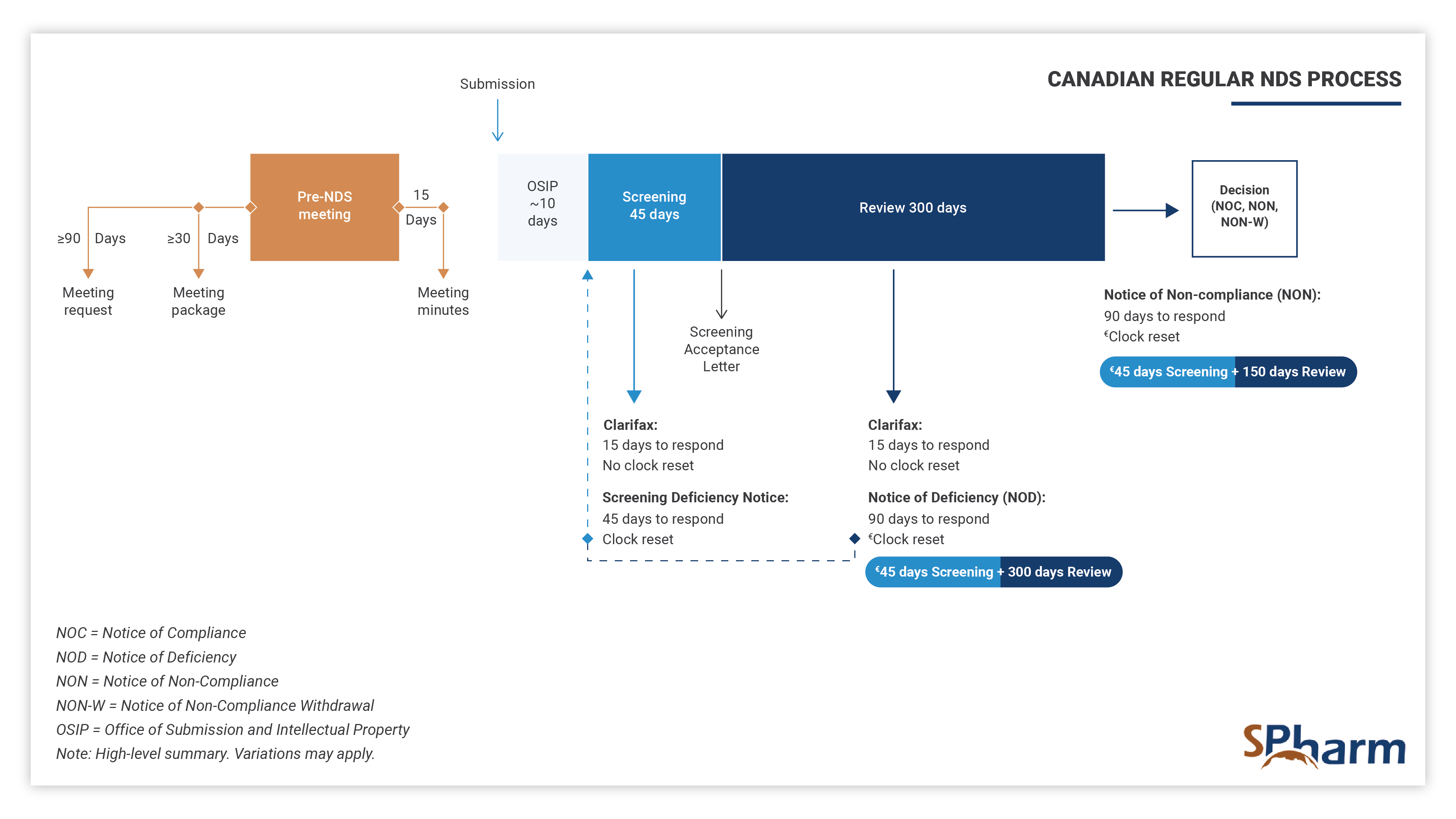
New Drug Submission Process in Canada

Current state of Health Canada regulation for cellular and gene

Clinical Trial Approval Process In Canada

Overview of Medical Device Clinical Trials - ScienceDirect

Canadian Medical Device Regulations 101

Clinical Trial Applications (CTA) - BlueReg Group

Canada 2018 Year in Review: Top 10 Medical Device Regulatory

Clinical Investigation - an overview

Health Canada Approval Process for Medical Devices: Step-by-Step Guide
/product/42/361962/1.jpg?6066)


![https://lp2.hm.com/hmgoepprod?set=quality%5B79%5D%2Csource%5B%2F4c%2Ff1%2F4cf1f72d5dd77c529fb9fe2b070c123a5ec51053.jpg%5D%2Corigin%5Bdam%5D%2Ccategory%5B%5D%2Ctype%5BLOOKBOOK%5D%2Cres%5Bm%5D%2Chmver%5B1%5D&call=url[file:/product/main]](https://lp2.hm.com/hmgoepprod?set=quality%5B79%5D%2Csource%5B%2F4c%2Ff1%2F4cf1f72d5dd77c529fb9fe2b070c123a5ec51053.jpg%5D%2Corigin%5Bdam%5D%2Ccategory%5B%5D%2Ctype%5BLOOKBOOK%5D%2Cres%5Bm%5D%2Chmver%5B1%5D&call=url[file:/product/main])