Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT } (i) What is the value of Z an ideal gas?(ii) For real gas what will be
4.6 (520) · $ 11.99 · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:compressibility factor z of a gas is given as z frac pv nrt
Click here👆to get an answer to your question ✍️ Compressibility factor- Z of a gas is given as Z- frac - pV - nRT - -i- What is the value of Z an ideal gas-ii- For real gas what will be the effect on value of Z above Boyle temperature
Answer in Molecular Physics Thermodynamics for Neilmar #278440

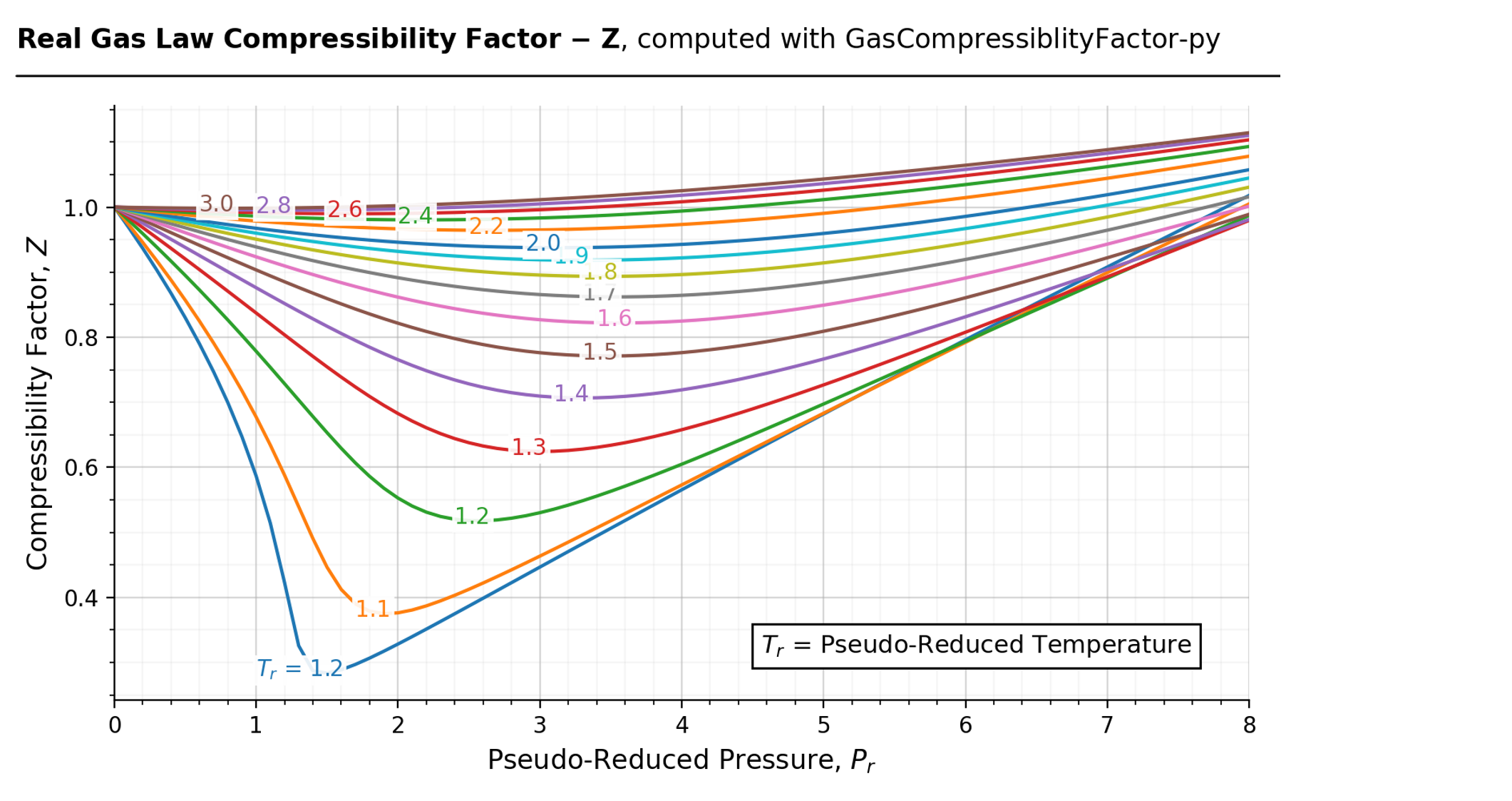
16.4: The Law of Corresponding States - Chemistry LibreTexts

Compressibility factor, Z of a gas is given as Z = pV / nRTi What

Non-Ideal Gas Behavior

Energies, Free Full-Text

Non-Ideal Gas Behavior Chemistry: Atoms First

DV (a) nb (c) – (n'a/v2) (d) - nb The given graph represent the
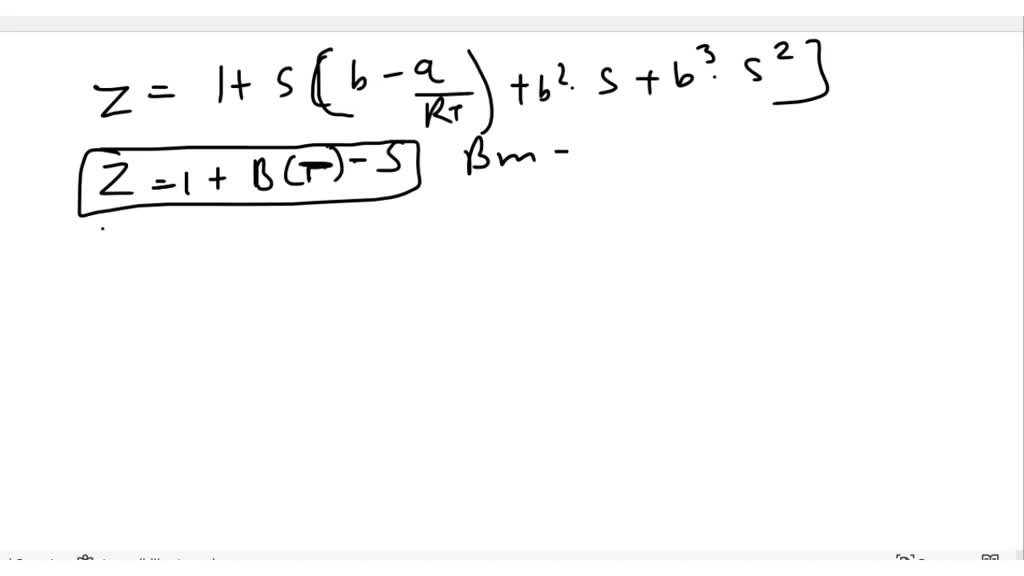
SOLVED: Derive the mathematical expression expressing the

Compressibility factor, Z of a gas is given as Z = pV / nRTi What

Energies, Free Full-Text

The compressibility factor of a gas is defined as Z=PV/nRT. The