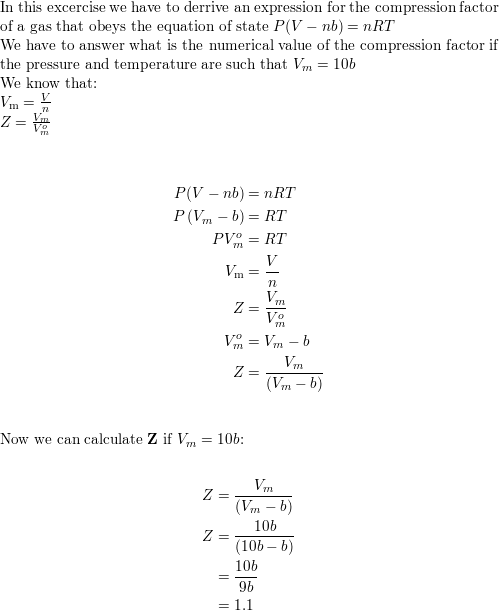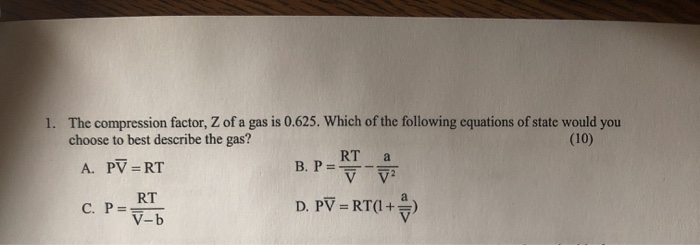Solved 9 Compression factor Z Use the van-der-Waals equation
5 (656) · $ 8.00 · In stock


Description of real gases: Compression factor

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
Thermo] Derivation of compressibility factor vs reduced pressure

6.3: Van der Waals and Other Gases - Physics LibreTexts

Non-ideal behavior of gases (article)

3.2 Real gas and compressibility factor – Introduction to

Compressibility factor, Z of a gas is given as Z= frac { pV }{ nRT

physical chemistry - Why do some gases have lower value of Z for a

If assertion is true but reason is false.

Chem II - Real Gases: Van der Waals (Liquids and Solids)

Van der Waals equation - Wikipedia

Van der Waals Equation - Derivation, Relation Between Ideal Gas

Physical Chemistry The Compression Factor (Z) [w/1 example

The compression factor (compressibility factor) one mole of a van