Draft Guidance Document: Applications for Medical Device Investigational Testing Authorizations
5 (570) · $ 14.50 · In stock

This draft guidance document reflects Health Canada’s current thinking on Investigational Testing Authorizations (ITA) for medical devices and may be subject to changes as policy develops. The document clarifies application requirements and processes, including pre-ITA meetings, format for an ITA application and filing requests for revisions to an ITA.
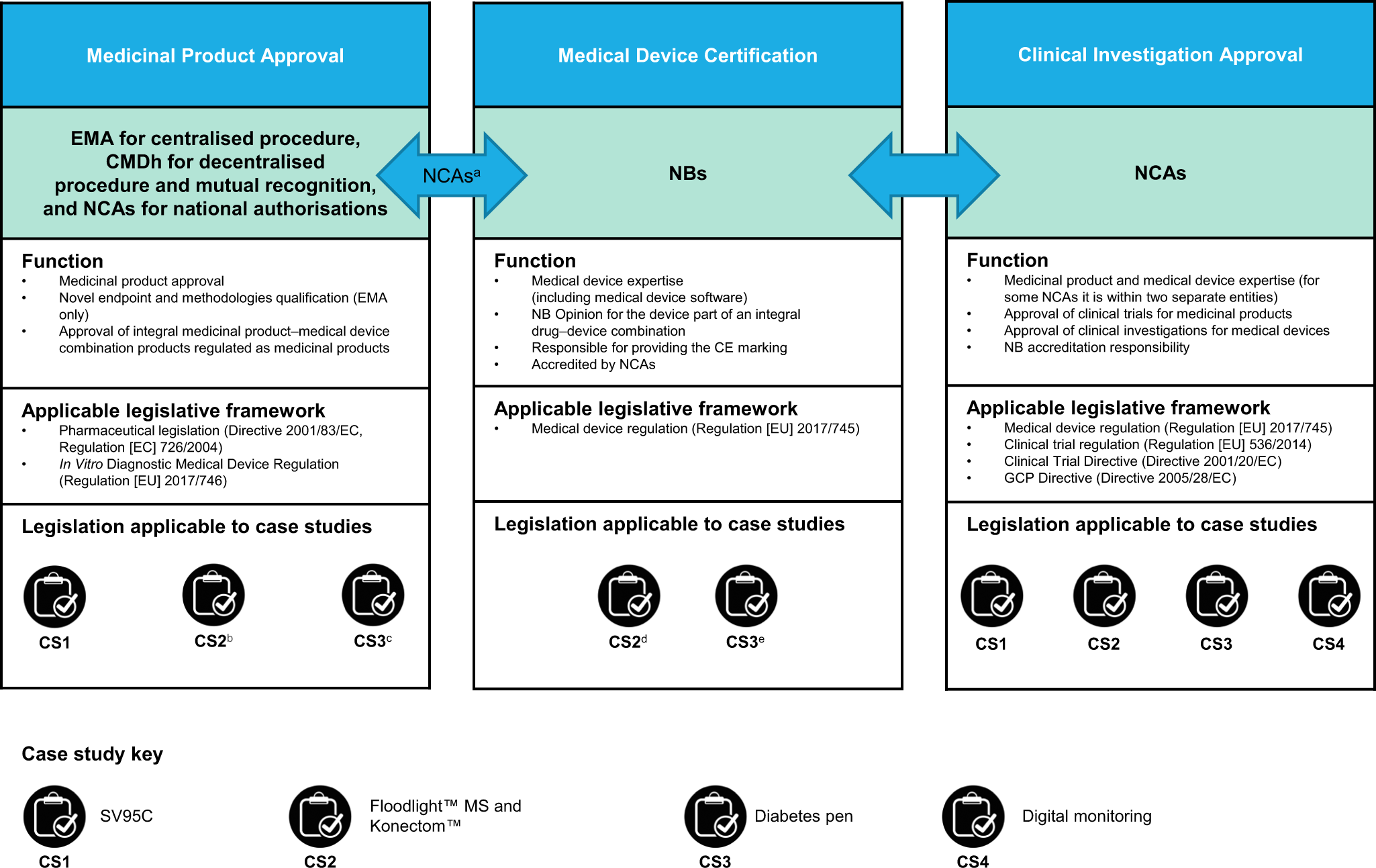
Evolving regulatory perspectives on digital health technologies for medicinal product development
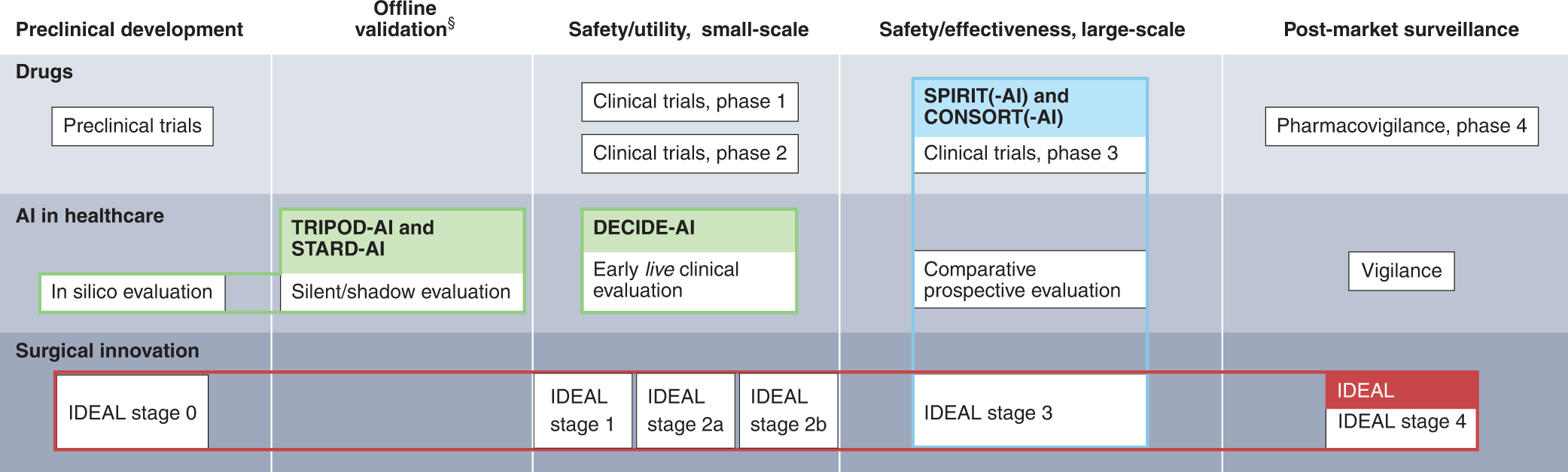
Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI
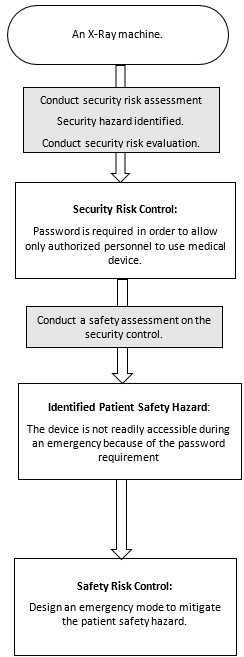
Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

Guidance Document: Pre-market Requirements for Medical Device Cybersecurity

Quality by design (QbD) approach in marketing authorization procedures of Non-Biological Complex Drugs: A critical evaluation - ScienceDirect

Draft guidance for determining medical device application type: Definitions
%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png?width=4250&name=(cover)%20A%20Complete%20Guide%20to%20Bringing%20a%20Medical%20Device%20to%20Market.png)
The Difference Between Intended Use and Indications of Use (And Why These Statements Are So Important)
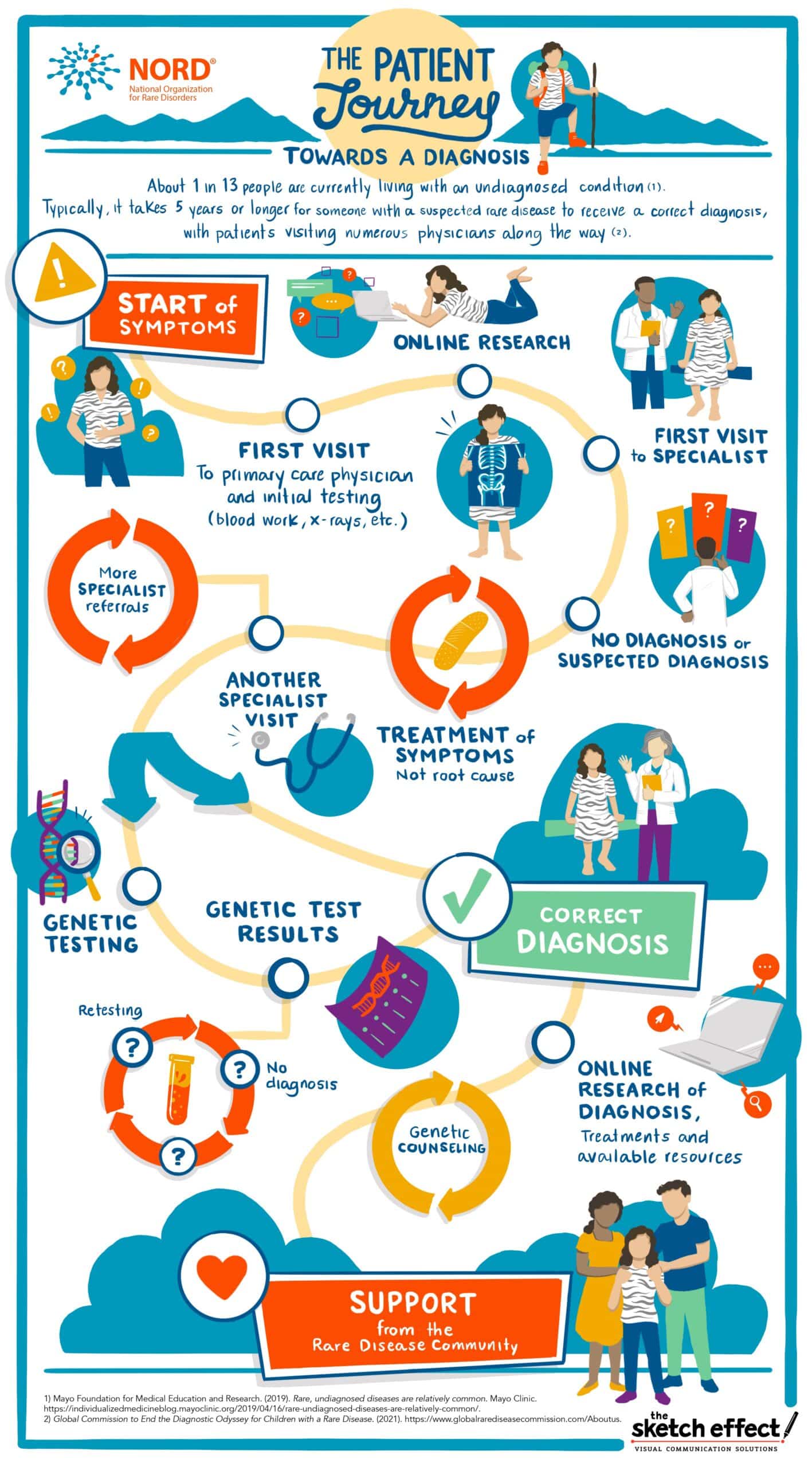
New Patient Journey Infographic Gives A Glimpse Into The Diagnostic Odyssey - National Organization for Rare Disorders
Decentralized Procedure for Marketing Authorization in EU (1-2) 5.

The evolution of Canada's medical device regulatory framework

Multi-Society Consensus Conference and Guideline on the Treatment of Gastroesophageal Reflux Disease (GERD) - A SAGES Publication