Microbiological Media Management - SOP & Guideline - Pharma Beginners
4.6 (536) · $ 14.00 · In stock

Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.

Validation Protocol To Determine The Shelf Life of Prepared Microbiological Media - Pharmaceutical Guidelines, PDF, Growth Medium

Guidelines for Temperature Control of Drug Products during Storage and Transportation (GUI-0069)

Qualifying your cleanroom

PDF) Microbiological Culture Media: A Complete Guide for Pharmaceutical and Healthcare Manufacturers

In-process Microbial Control During Aseptic Processing (High-Risk)

50+ Free & Easy SOP Templates (Sample SOPs to Record Standard Procedures), Process Street

EN ISO 11133 and Water for the Preparation and Performance Testing
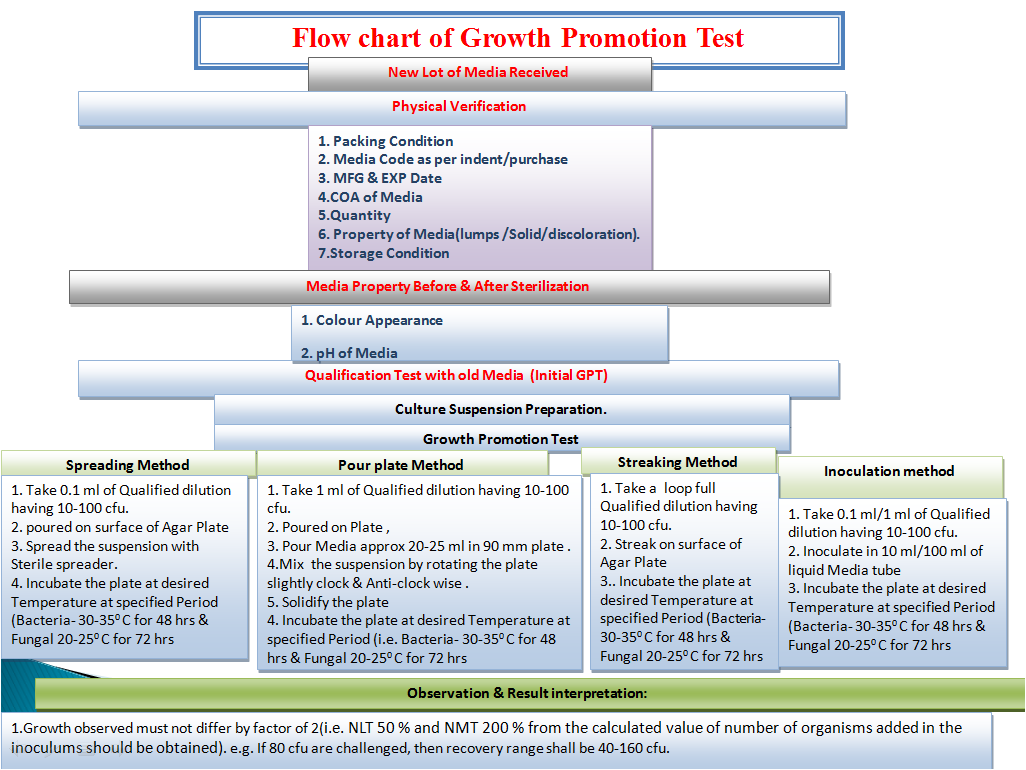
Growth promotion test 2023
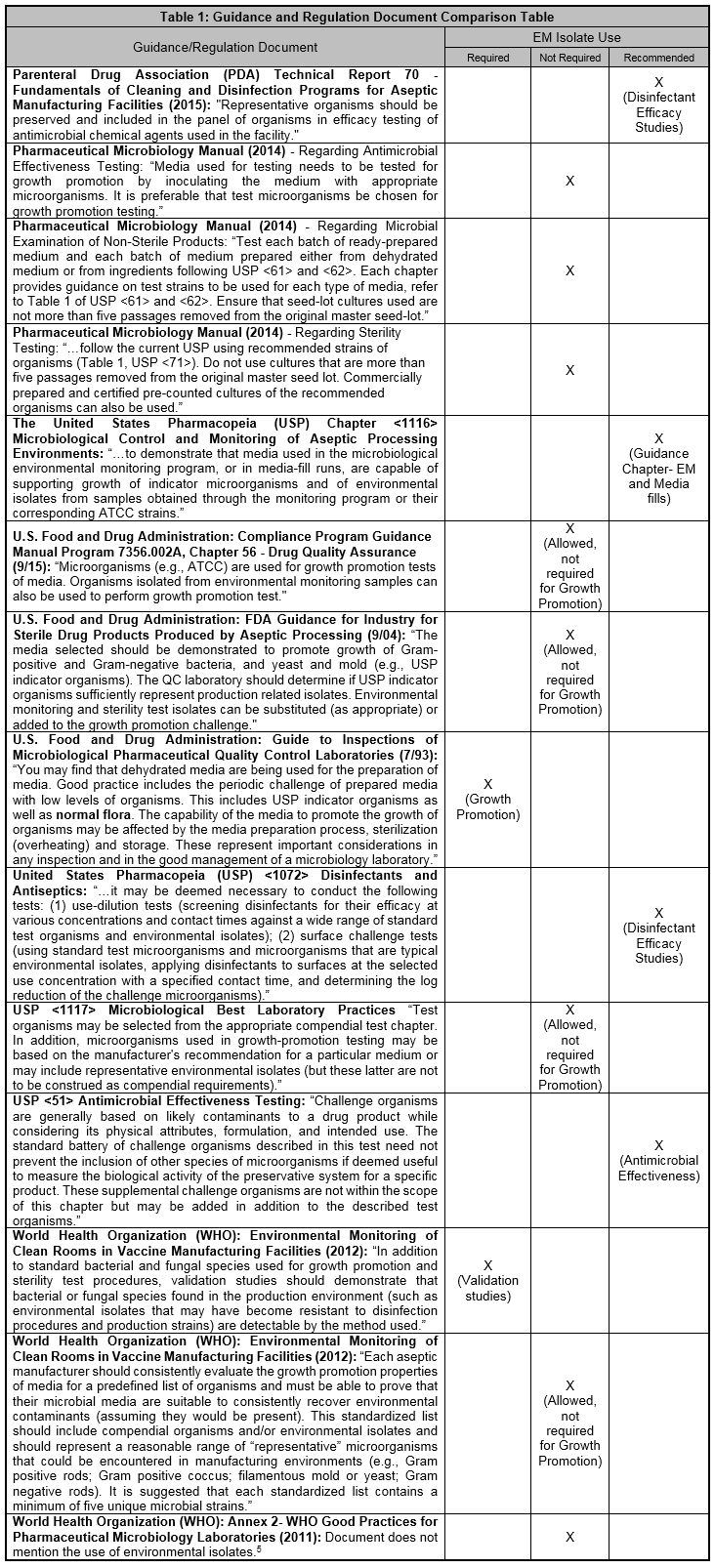
Environmental Isolates What's The Proper Use Of In-House Cultures







