Metals, Free Full-Text
4.9 (269) · $ 13.00 · In stock
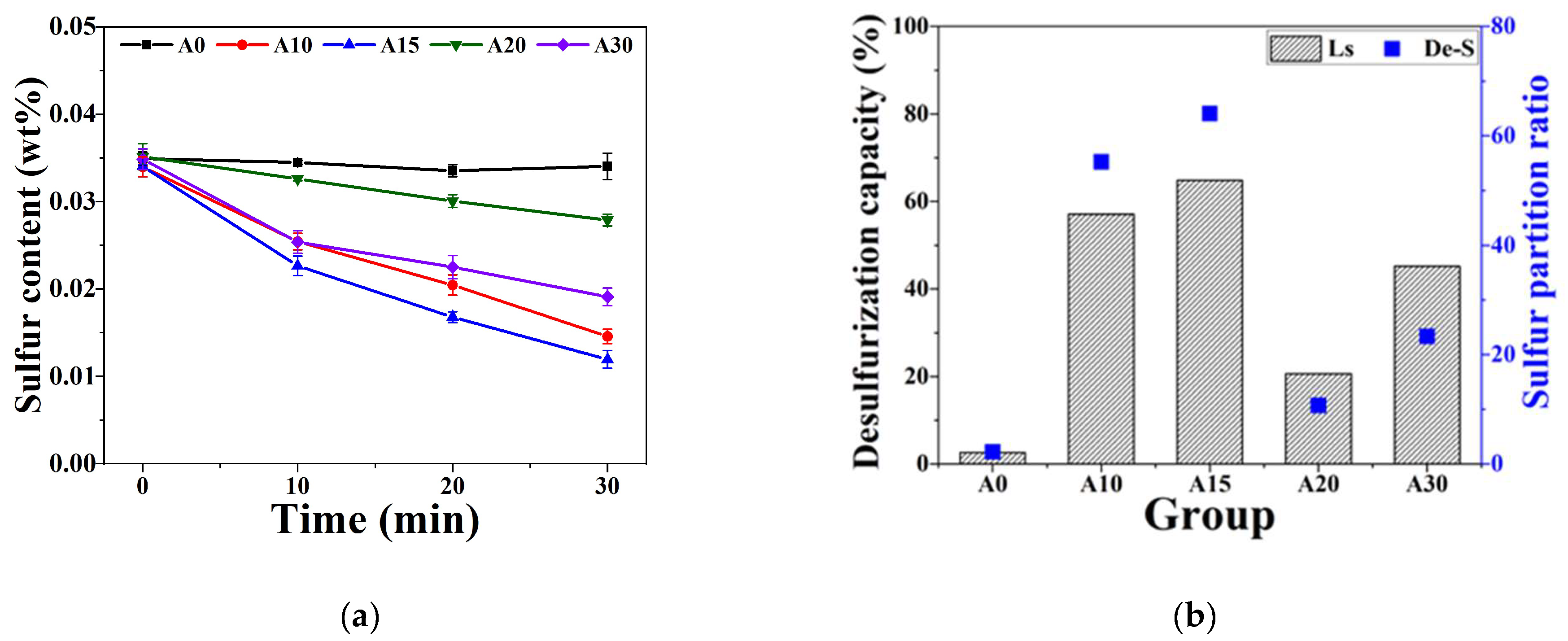
In response to the development of low-carbon smelting technology, reducing the use of fluor-containing materials in desulfurizers is an important research topic. The development of new-generation KR (Kambara Reactor) desulfurizers is shifting towards a higher Al2O3 content rather than CaF2, yet there is currently an absence of thorough and comprehensive mechanisms for desulfurization. Consequently, this research provides an extensive comparison using a specially constructed small-scale KR desulfurization hot model test, alongside FactSage simulation and SEM analysis (of desulfurization process). The findings indicate that at 1400 °C, for the desulfurization of molten iron, the capacity for desulfurization initially increases and then diminishes as the Al2O3 content in the KR desulfurizer rises. With Al2O3 content in the desulfurizer below 22 wt.%, the phase composition predominantly consists of C3A, employing a solid(slag)–liquid(metal) diffusion method for desulfurization. The optimal desulfurization capacity (Ls: 64.1) is observed when the Al2O3 content is 15 wt.%, attributed to the simultaneous presence of CaO particle precipitation and C3A. However, as the Al2O3 content reaches 20 wt.%, all the oversaturated CaO integrates into C3A, leading to a reduction in Ls from 64.1 to 10.7, thereby diminishing the desulfurization capacity by approximately sixfold. When Al2O3 exceeds 22 wt.%, the phase composition transitions from the C3A to C12A7 phase, and the desulfurization approach shifts from solid(slag)–liquid(metal) to liquid(slag)–liquid(metal) diffusion, with Ls decreasing to 23.4. This reduction is due to C12A7’s lower sulfur capacity compared to C3A and the absence of saturated CaO particle precipitation. Therefore, for Al2O3 to effectively replace fluorite in KR desulfurizers, a higher presence of C3A phases and CaO particle precipitation are essential. The desulfurizer must contain over 65 wt.% CaO and maintain Al2O3 levels at 10~16.2 wt.%.

Metals and Mining
Transactions of American Society for Metals 1953: Vol 45 : Free Download, Borrow, and Streaming : Internet Archive
Metals, Free Full-Text

Wills Scrap Metal Recycling & Son's

SOLUTION: Free electron theory of metals - Studypool

Metals, Free Full-Text
Metals, Free Full-Text

Nonmetal - Wikipedia

Elemental analysis of commercial zirconia dental implants - Is “metal-free” devoid of metals? - ScienceDirect

Periodic Table Metals and Non-Metals

Pure Copper Powder for Additive Manufacturing |Special Site of JX Metals, Copper Powder
Metals, Free Full-Text, simulation unclogger
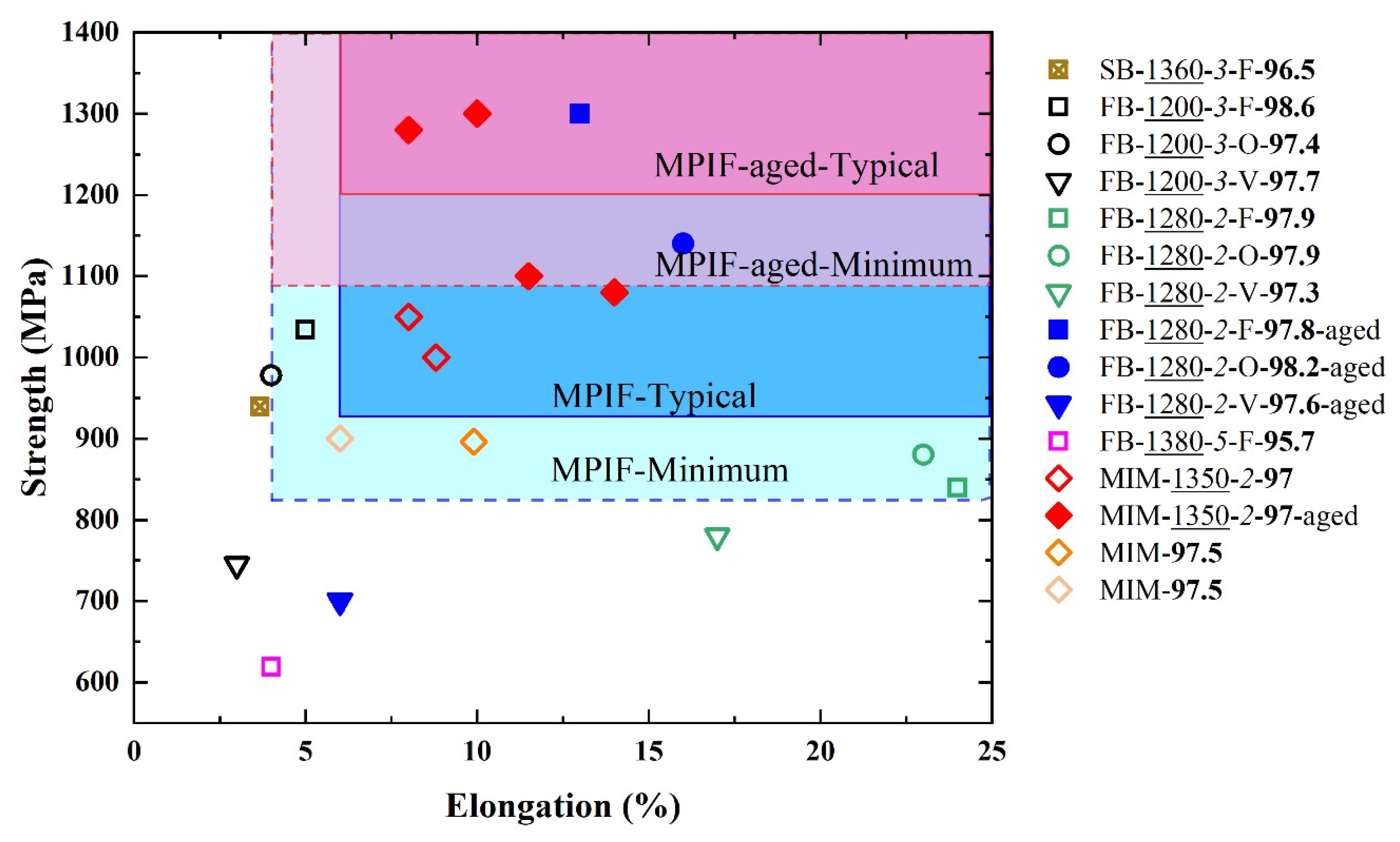
Metals, Free Full-Text
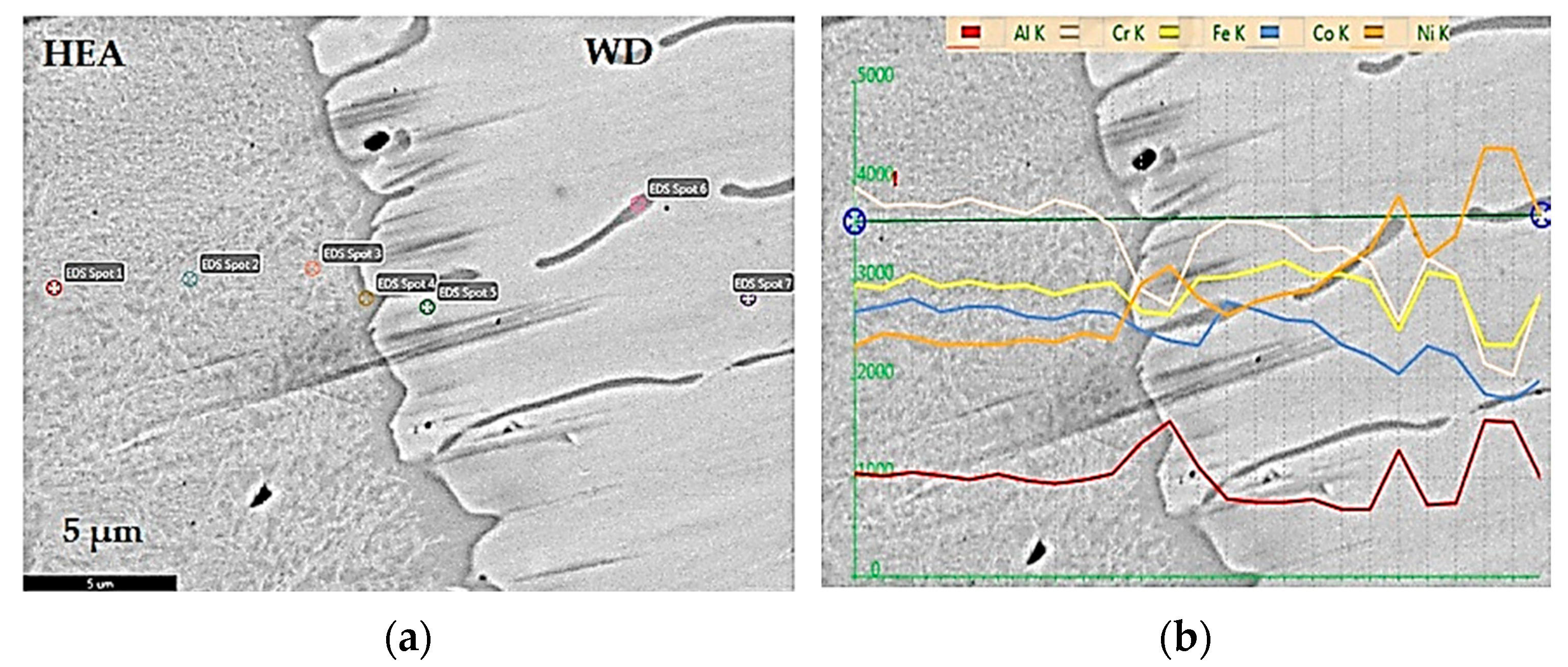
Metals, Free Full-Text
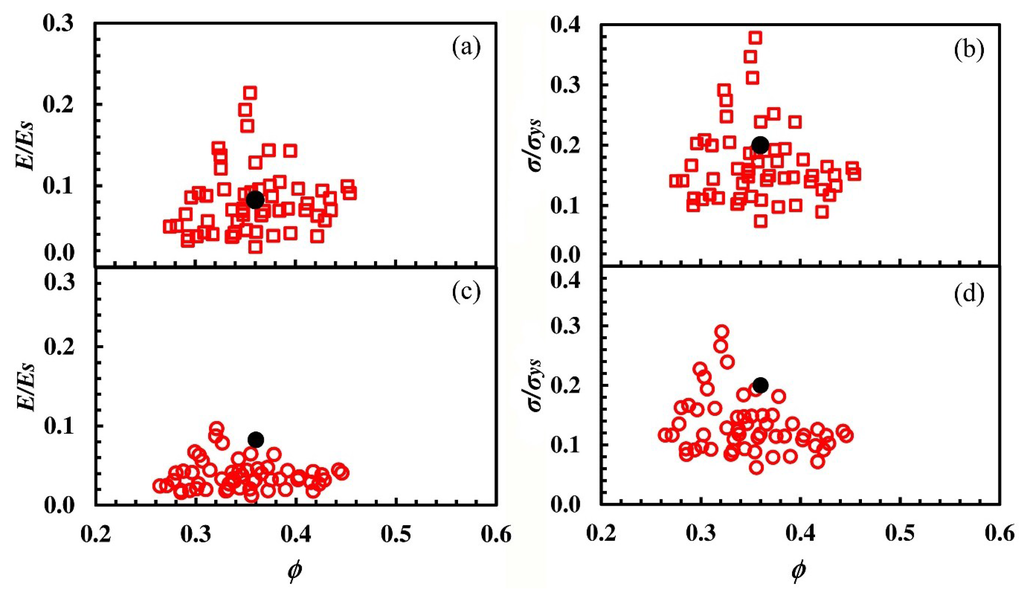
Metals, Free Full-Text





:max_bytes(150000):strip_icc()/GettyImages-677164657-589794cc3df78caebc0adc81.jpg)