SOLVED: Qussion 2 [14 Marks] 2.1 Consider an equation of state for gas given by 2 =1+ Vm Va where B and € are constants and Z is the compressibility (compression) factor.
4.7 (468) · $ 15.50 · In stock
![SOLVED: Qussion 2 [14 Marks] 2.1 Consider an equation of state for gas given by 2 =1+ Vm Va where B and € are constants and Z is the compressibility (compression) factor.](https://cdn.numerade.com/ask_images/1b609a8cb53e406096ad8d4adacdcfe3.jpg)
VIDEO ANSWER: The equation of state is 1 plus b, divided by v m plus c and divided by v m square, which is the answer to the question given here. Okay, let's get to it. The equation can be considered here. Number One point. We need to calculate the
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Gaseous State Ex. 1 & 2 PDF
![SOLVED: Qussion 2 [14 Marks] 2.1 Consider an equation of state for](https://cdn.numerade.com/ask_previews/859e0331-cce9-44b4-bfef-6a493c130ec8.gif)
SOLVED: Qussion 2 [14 Marks] 2.1 Consider an equation of state for

Solution manual fundamentals of fluid mechanics (4th edition)

SOLVED: Question 2 2.1 Consider an equation of state for a gas

Solutions Manual Fundamentals of Fluid Mechanics 3Rd and 4Th
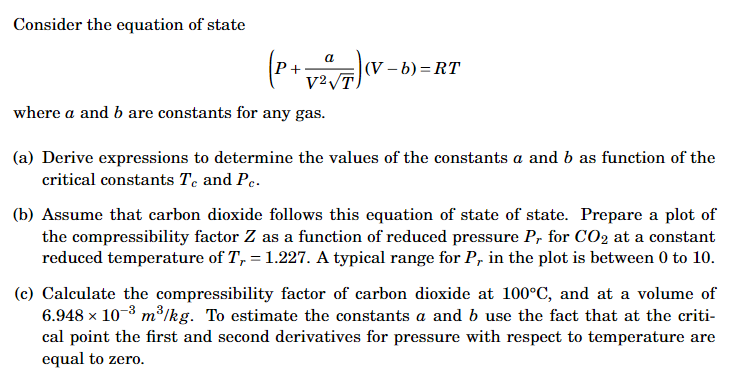
Consider the equation of state (P+V2Ta)(V−b)=RT where
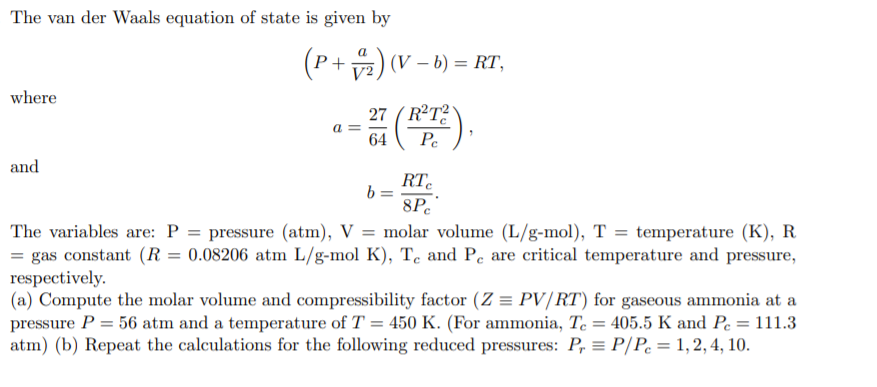
Solved a = The van der Waals equation of state is given by

PROBLEMS AND SOLUTIONS ON MECHANICS

PDF) Physical Chemistry by P Bahadur







