Solved 3.30 The density of water is 1.00 g/mL at 48C. How
5 (733) · $ 15.00 · In stock

What is the volume of a solution, in mL, of sucrose, (C12H22O11
Relation between water holding capacity (WHC) and swelling

Plants, Free Full-Text
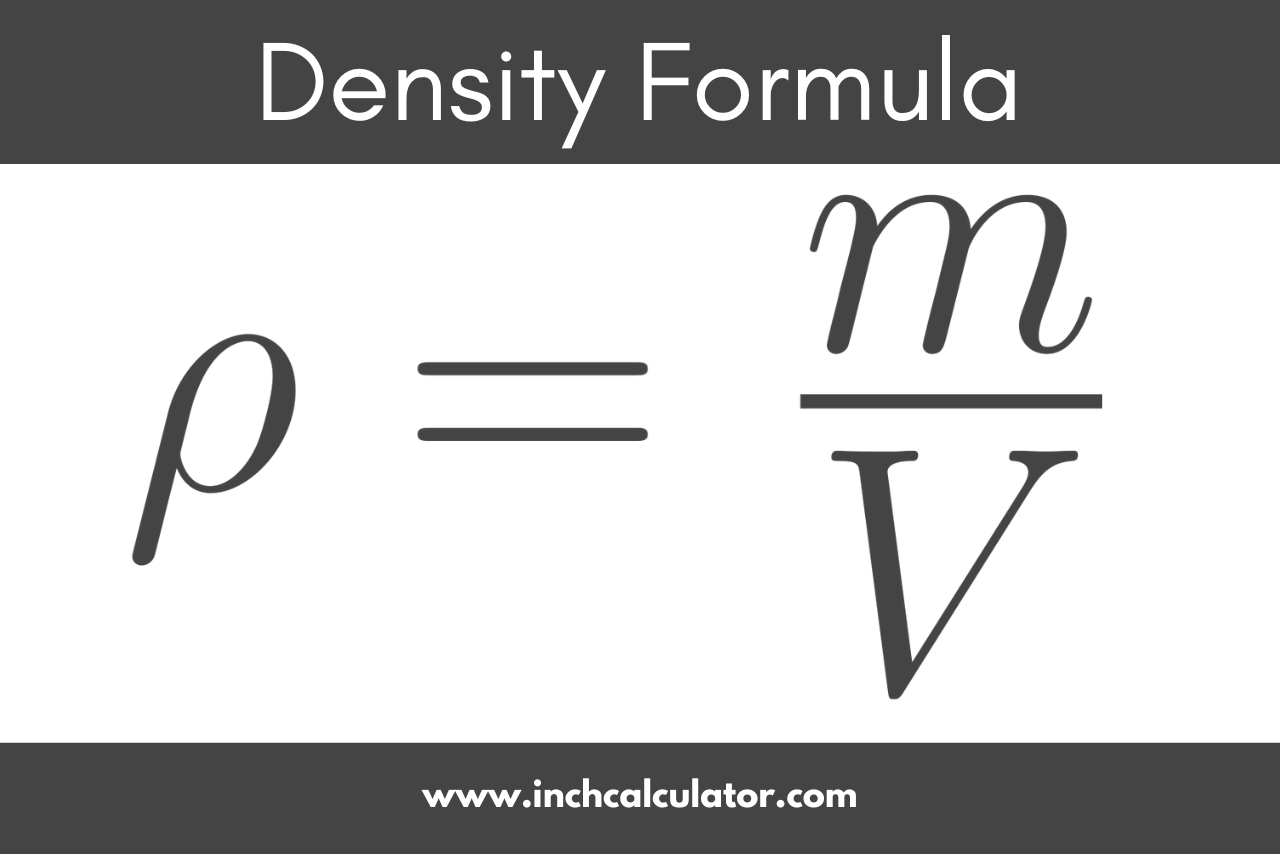
Density Calculator - Inch Calculator
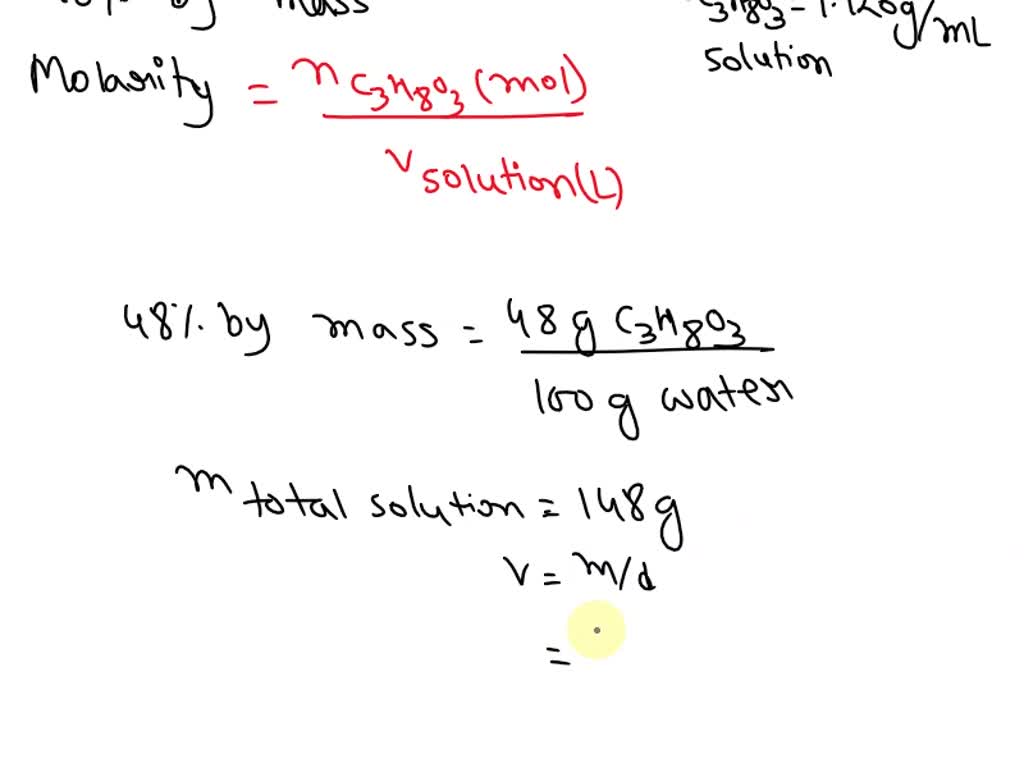
SOLVED: An aqueous solution of glycerol, C3H8O3, is 48.00

Solved Complete the following problems. Use significant
If oil is heavier than water, how does it float on top of water? Why doesn't gravity affect the heavier substance? - Quora

Theoretical and Experimental Characterization of Adsorbed CO and NO on γ-Al2O3-Supported Rh Nanoparticles
When it is given that the density of an aqueous solution of sodium

Solved example, consider the density of water, which is

SOLVED: a) An aqueous solution is 30.0% hydrofluoric acid (HF
If density of water at 4°c is 1g cm^-3 . Then what is the value of
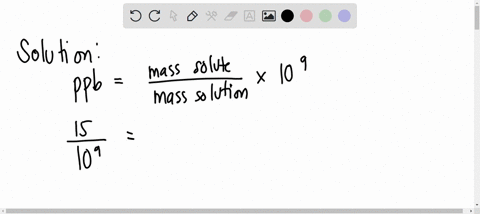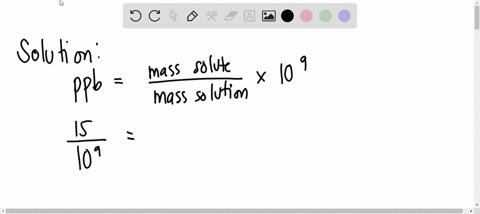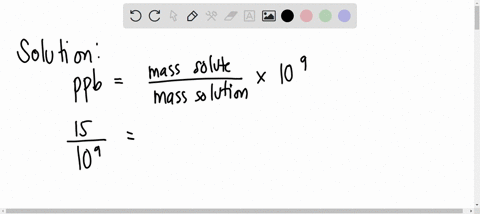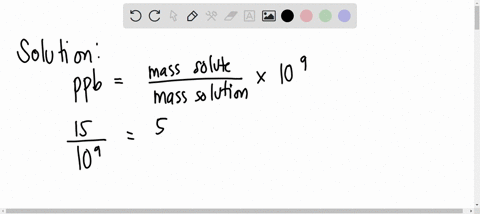
SOLVED: A water sample contains the pollutant chlorobenzene with a concentration of 17 ppb (parts per billion) by mass. What volume of this water contains 5.41×10^2 mg of chlorobenzene? (Assume a density







