Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is
4.9 (637) · $ 16.00 · In stock

Answer to Solved Problem 3 (Phase changes) A 35 g ice cube at 0°C is
What is the amount of heat required to heat 1kg of ice from -10°c to 0°c? - Quora

Answered: 4. How much energy is removed from…

Solved An ice cube (mass 30 g) at 0 degree C is left sitting
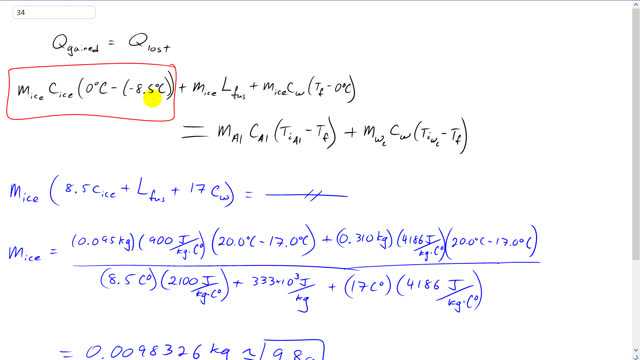
Giancoli 7th Edition, Chapter 14, Problem 34

Solved A 53.3-g ice cube is initially at 0.0°C. (a) Find the

Ice - Wikipedia

Temperature Change and Heat Capacity
What is a graph showing a phase change in temperature when ice is heated from -10C to over 100C? - Quora
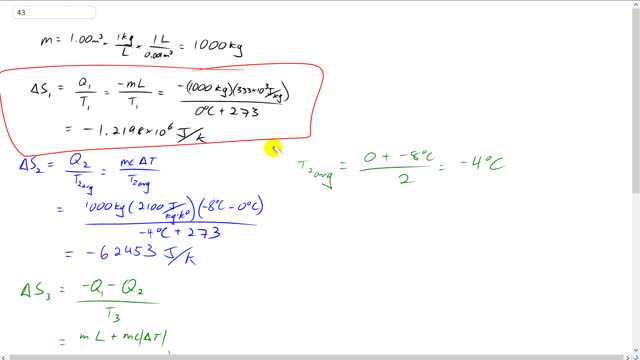
Giancoli 7th Edition, Chapter 15, Problem 43
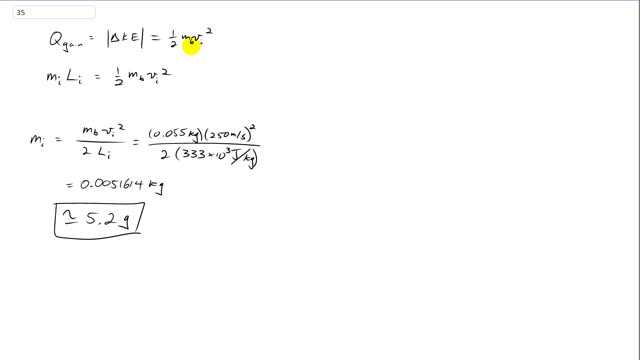
Giancoli 7th Edition, Chapter 14, Problem 35
What is the amount of energy required to change 10g of ice at 0°c to water at 20°C? - Quora
8.What is the entropy change when one mole of ice is converted into water at 0 degree Celsius? (the entropy change for the conversion of ice to liquid water is 6.0 kJ







