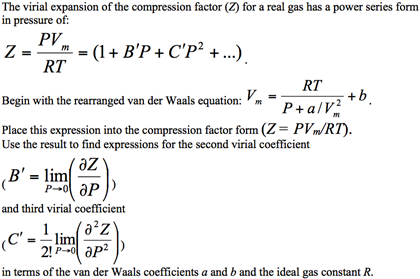
UNUB At Boyle temperature, the value of compressi factor Z has a
5 (705) · $ 13.99 · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:unubat boyle temperature the value of compressifactor z has a value of one over a
Click here👆to get an answer to your question ✍️ UNUB At Boyle temperature- the value of compressi factor Z has a value of one over a wide range of pressure- This is due to the fact that in the van der Waals equation -1- The constant a is negligible and not b -2- The constant b is negligible and not a -3- Both the constant a and b are negligible -4- Attraction balances repulsion

qph.cf2.quoracdn.net/main-thumb-137510504-200-syqq
![Physical Chemistry The Compression Factor (Z) [w/1 example]](https://i.ytimg.com/vi/3ta9OUAC4IY/maxresdefault.jpg)
Physical Chemistry The Compression Factor (Z) [w/1 example]

A LEVEL Heat and Modern 2016, PDF, Thermometer

PDF) Effect of Temperature and Z-Factor on Casing Designusing Kick

The compressibility factor for a real gas at high pressure is

and two-phase flow in singular geometries and safety relief valves

UNUB At Boyle temperature, the value of compressi factor Z has a value of one over a wide range of pressure. This is due to the fact that in the van der

and two-phase flow in singular geometries and safety relief valves
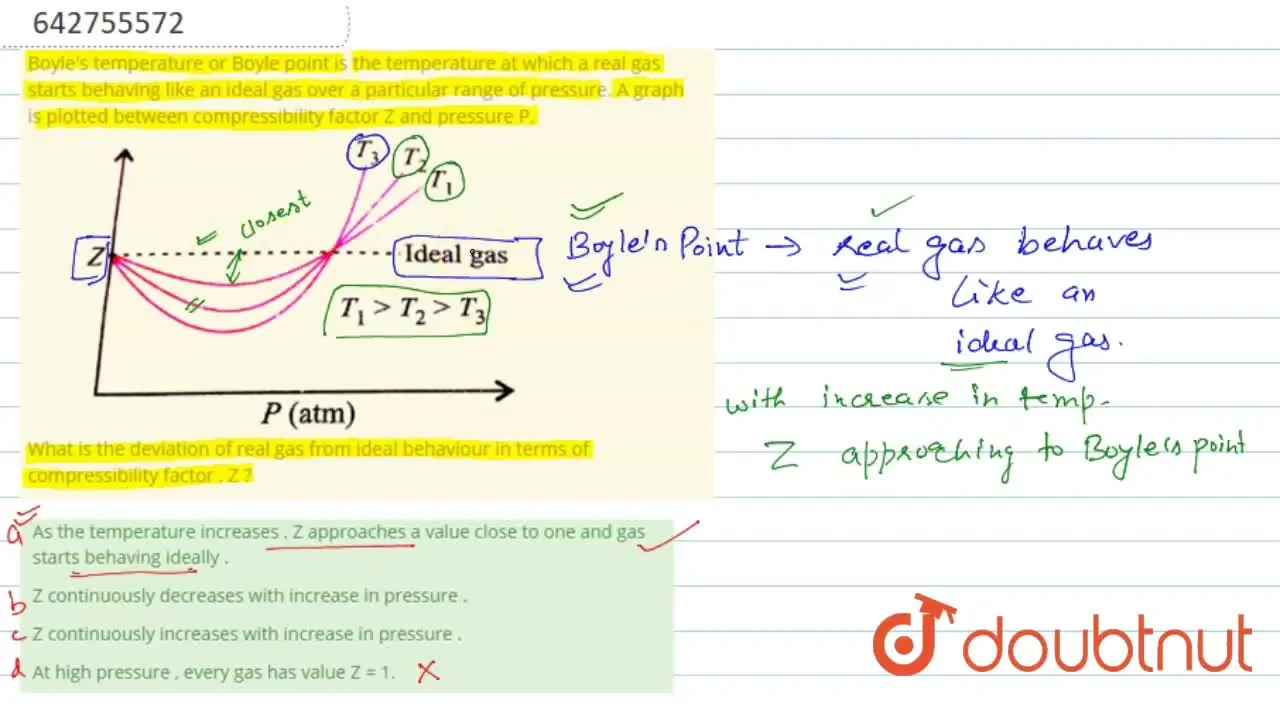
Z continuously increases with increase in pressure .

Solved 2. By definition, the compression factor of an ideal

Chemistry_1 - Flipbook by NOWFIYA N

At Boyle's temperature , compressibility factor Z for a real gas is