Welcome to Chem Zipper.com: The compressibility factor for 1 mole of a van der Waals gas at 0oC and 100 atm pressure is found to be 0.5. Assuming that the volume of
4.5 (219) · $ 18.50 · In stock


What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

Welcome to Chem Zipper.com: THE STATE OF MATTER

What is the compressibility factor (Z) for 0.02 mole of a van der Waal

What is the compressibility factor Z for 0.02 mole of a van der waal's gas at pressure of 0.1 atm. Assume the size of gas molecule is negligible. Given: RT =20 L

The compression factor (compressibility factor) for 1 mol of a van der
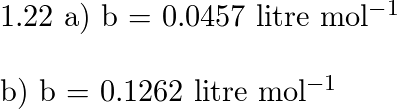
a) A certain gas obeys the van der Waals equation with $a =

1148 questions with answers in GAS
List references from the University of Geneva Physical Chemistry reference database

1148 questions with answers in GAS

Kirkwood–Buff-Derived Force Field for Peptides and Proteins: Applications of KBFF20

Graeff's experiments and 2LoD: Replication and Implications
Université de Genève - Groupe du Professeur Andreas Hauser

The compressibility factor 1 mole of vanderwaal gas 0^{o}C, and 100 atm pressure is found to be 0.5, then calculate the vander Waals constant a. Assuming: that the volume of gas molecule

The compression factor (compressibility factor) one mole of a van der Waals gas 0°C and 100 atm pressure is found to be 0.5. Assuming that the volume of a gas molecule is







