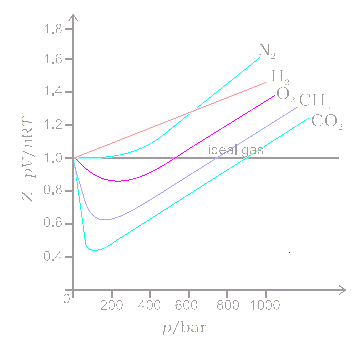
Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an
4.6 (222) · $ 17.99 · In stock

![Bengali] The compresibility factor (Z) of one mole of a van der waals](https://static.doubtnut.com/ss/web-overlay-thumb/2552448.webp)
Bengali] The compresibility factor (Z) of one mole of a van der waals

Real-gas z-factor, as attributed to Standing and Katz, 9 plotted as a
Van der waals equation: Derivation, Explanation

Energies, Free Full-Text
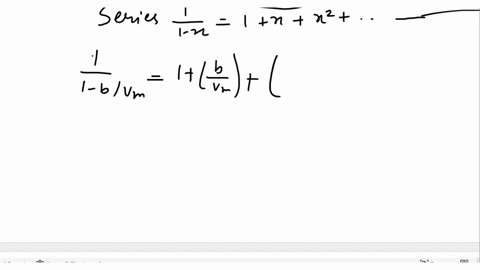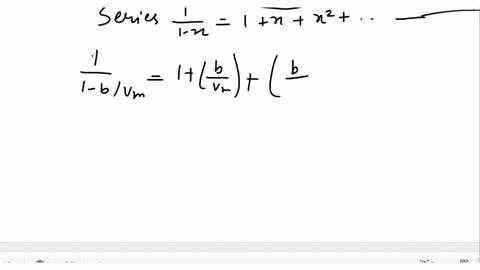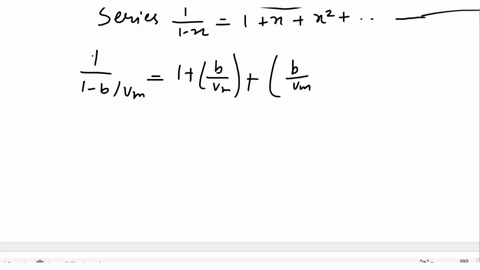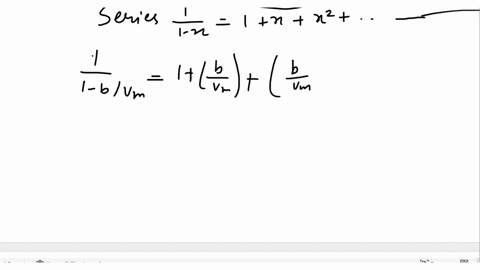
SOLVED: Derive the mathematical expression expressing the compressibility factor Z of a real gas depending on the reduced variables; Explain in detail how the volume of the actual gas at a given

Compressibility Factor Calculator

cdn.kastatic.org/ka-perseus-images/854dcada2b7466c

The compressibility factor (Z) of one mole of a van der Waals' gas of negligible 'a ' value is:1dfrac{bp}{RT}1+dfrac{bp}{RT}1-dfrac{bp}{RT}

Thermodynamics: An Engineering Approach - 5th Edition - Part II by 黑傑克 - Issuu





![Real Gas Behavior The Compression Factor (Z) [Example #2]](https://i.ytimg.com/vi/Z5NsvRPZT6I/maxresdefault.jpg)

