For H(2) gas, the compressibility factor,Z = PV //n RT is
4.9 (334) · $ 14.50 · In stock

For H(2) gas, the compressibility factor,Z = PV //n RT is

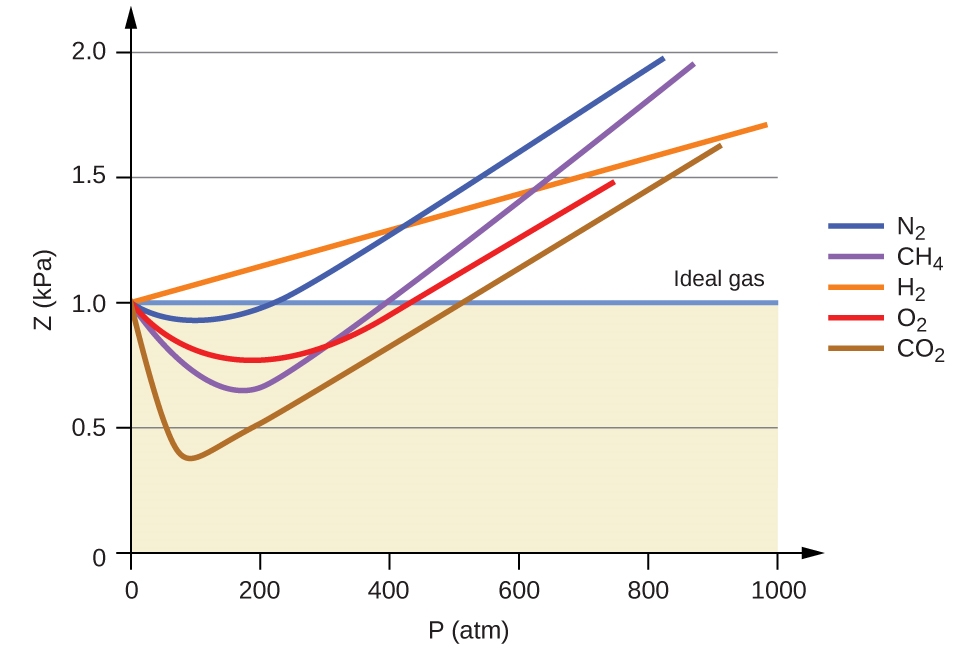
PV Compressibility factor Z= nRT is plotted against pressure : N. Ideal gas What is the correct order of liquefiability of the gases shown in the above graph? H
![Kannada] For H(2) gas, the compressibility factor, Z = pV //nRT is](https://static.doubtnut.com/ss/web-overlay-thumb/8871461.webp)
Kannada] For H(2) gas, the compressibility factor, Z = pV //nRT is

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks
The given graph represent the variation of z compressibility factor z=pv/nRT versis p fpr three real gases A,B,C identify only incorrect statement
2.8 – Real/Non-Ideal Gas Behaviours – General Chemistry for Gee-Gees

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
![ANSWERED] Q 32 Compressibility factor Z of a gas is given as Z pV nRT - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20210628105133699196-2457313.jpg)
ANSWERED] Q 32 Compressibility factor Z of a gas is given as Z pV nRT - Kunduz

For H(2) gas, the compressibility factor,Z = PV //n RT is
What is the value of compressibility factor in terms of vander waal cons†an t at different conditions of pressure and volume?Why is Z>1 for H2 and He gas

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Chem II - Real Gases: Van der Waals (Liquids and Solids)







