The compressibility factor is Z = PV/R_g T. Evaluate
4.5 (399) · $ 24.99 · In stock

Answer to The compressibility factor is Z = PV/R_g T. Evaluate

Gas compressibility factor Z: Ideal gas vs Real gas

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea

Gas compressibility factor Z: Ideal gas vs Real gas

Determine Compressibility of Gases

1. The compressibility factor, z, is the ratio of
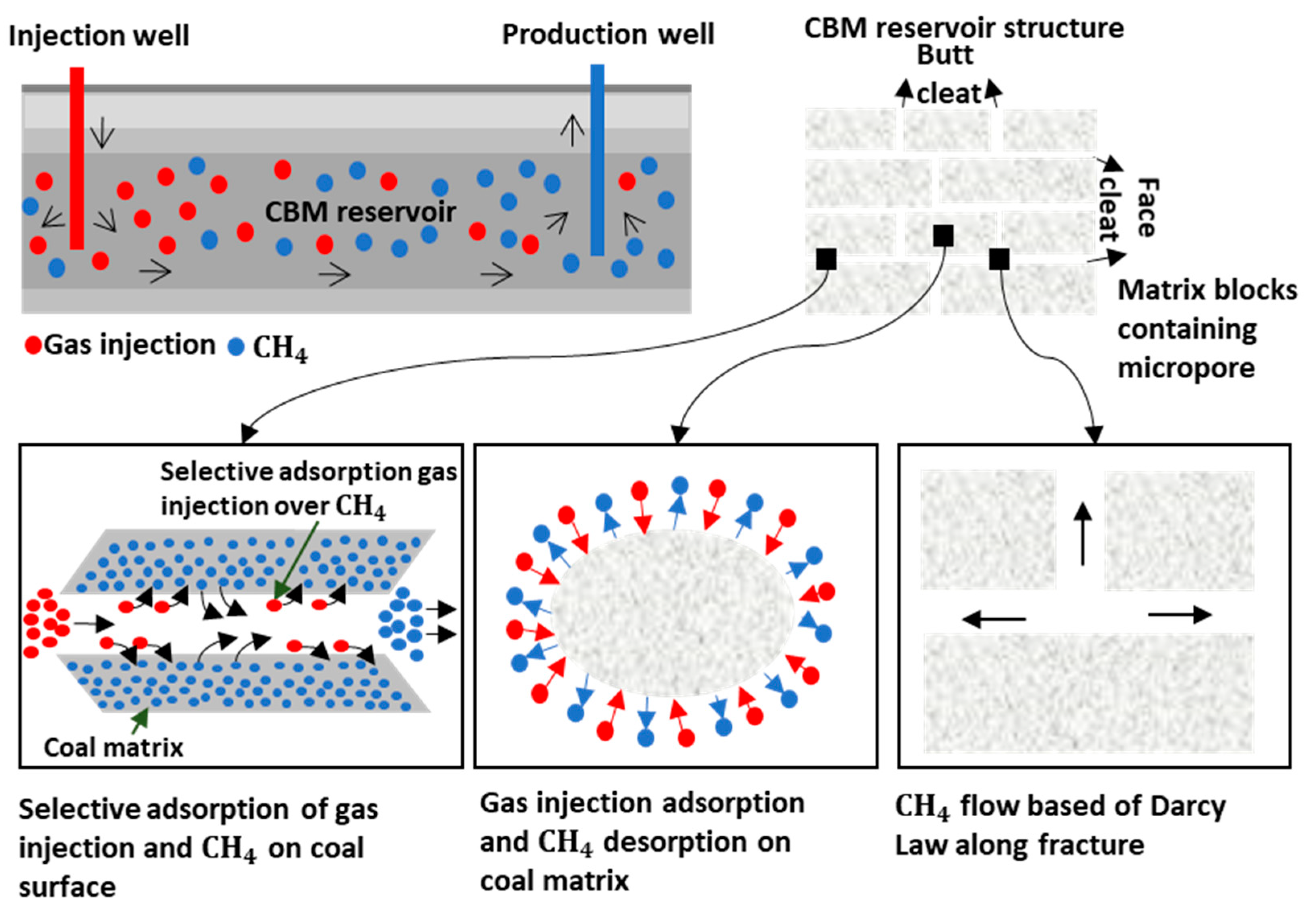
Gases, Free Full-Text
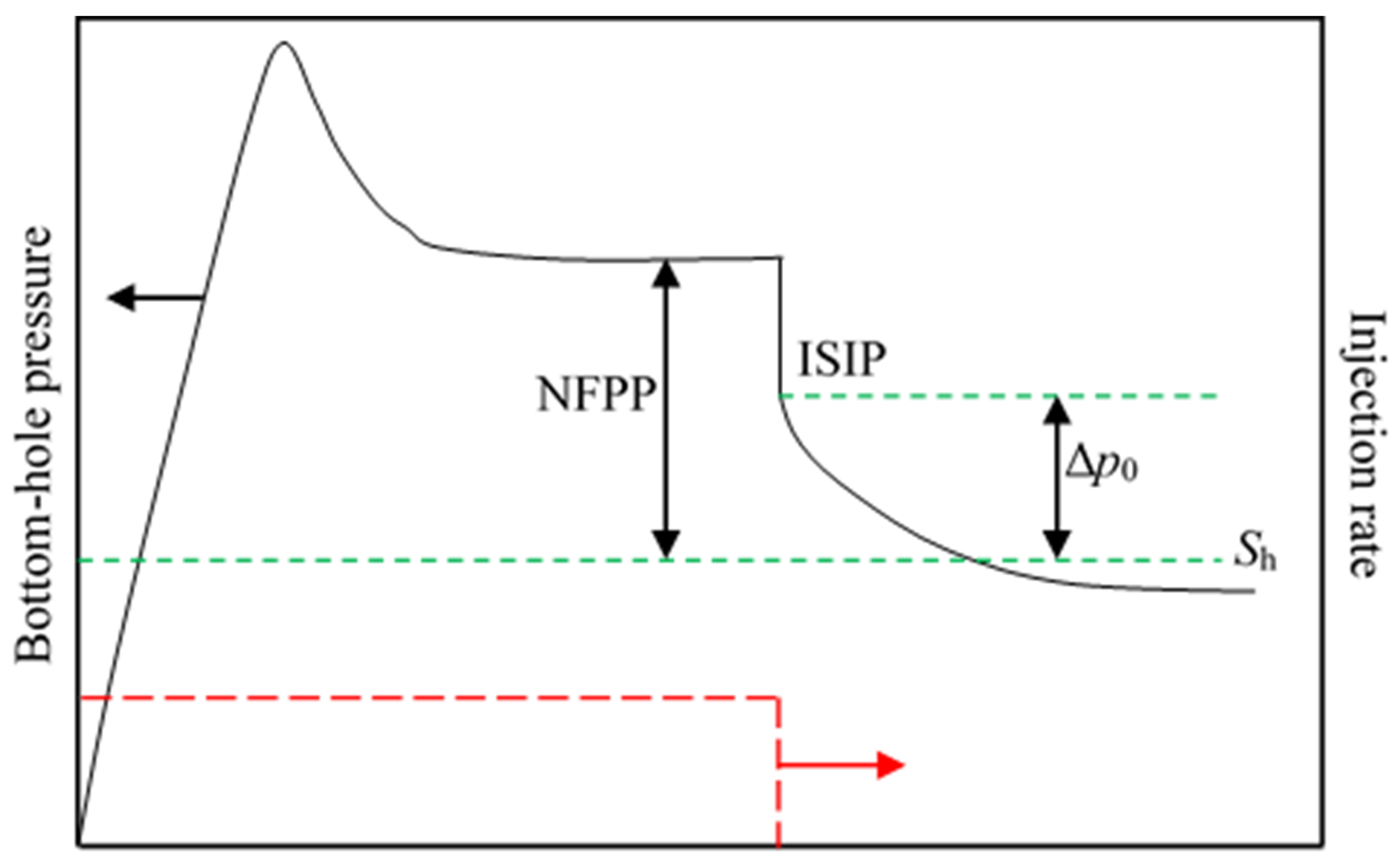
Processes, Free Full-Text

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect

Compressibility factor Z - Gaseous State

PDF) Predicting the compressibility factor of natural gases containing various amounts of CO2 at high temperatures and pressures






