thermodynamics - Variation of compressiblity factor with
4.9 (198) · $ 5.00 · In stock

While I certainly understand the order of temperatures, I can't find a reason for the curves to intersect at one common point. Why do the curves intersect at one point? or do they really intersect

Acentric Factor - an overview

Compressibility Factor Z Important Concepts and Tips for JEE Main

Thermodynamics Lecture 7: Compressibility
![Telugu] The variation of compressibility factor (Z) with pressure (p](https://d10lpgp6xz60nq.cloudfront.net/physics_images/ARH_5Y_SP_03_05_19_02_E03_008_S01.png)
Telugu] The variation of compressibility factor (Z) with pressure (p
JEE - Compressibility Factor Important Concepts and Tips

Variation of Helmholtz free energy (F) with the Horizon radius

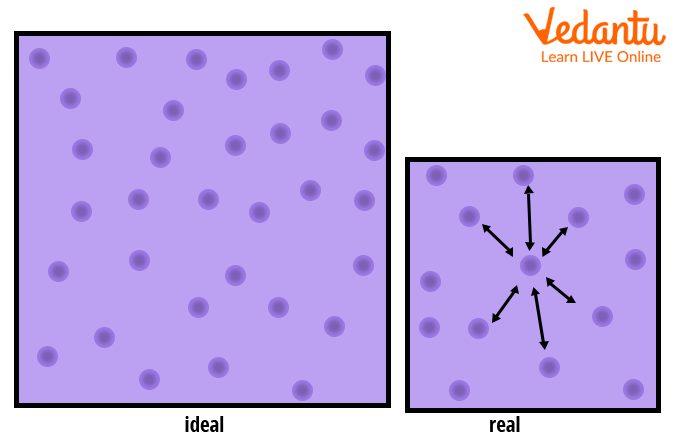
3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics
The given graph represent the variations of compressibility factor

Variation of deviation in isentropic compressibility, Δks, with mole

Compressibility factor Z is plotted against pressure p for four different gases A,B, C D. The correct order of critical temperature of the gases shown in the above graph is

Compressibility Factor Charts - Wolfram Demonstrations Project

Energies, Free Full-Text

Boyle's temperature or Boyle point is the temperature which a real gas starts behaving like an ideal gas over a particular range of pressure. A graph is plotted between compressibility factor Z

Figure . Compressibility factor Z = PV/NkT of the SW fluid plotted

Real Gases Introductory Chemistry