At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to
5 (597) · $ 6.50 · In stock
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to
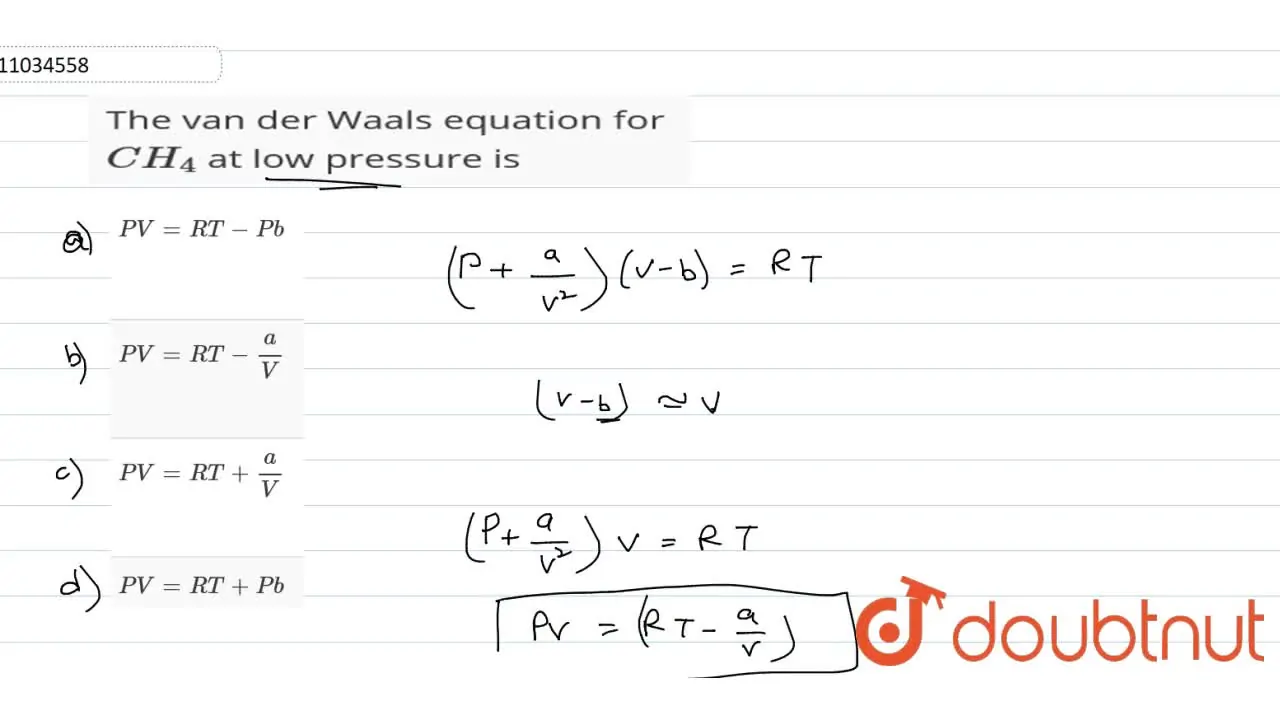
The van der Waals equation for CH(4) at low pressure is

Compressibility factor (Z) a real gas moderately low pressure is
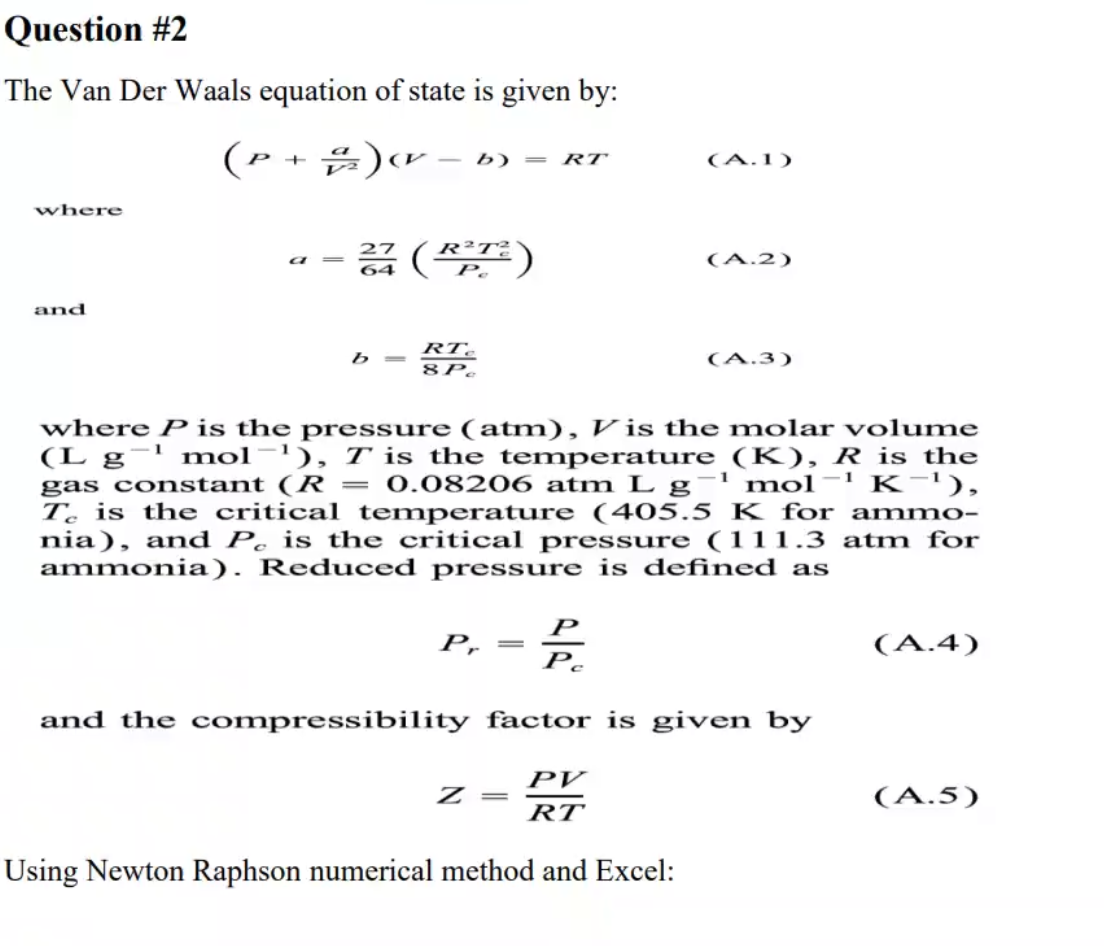
Solved The Van Der Waals equation of state is given by

Solved 1. Assume that water vapor is a Van der Waals gas. a

At low pressure, the van der Waals equation is reduced to

Why do we use the ideal gas equation when instead van der Waals
Why is the term that corrects for volume, negative and the term
What is the unit of a and b in van der Waals' equation if it is

The van der Waals and Redlich-Kwong Equations, which
What is the unit of a and b in van der Waals' equation if it is
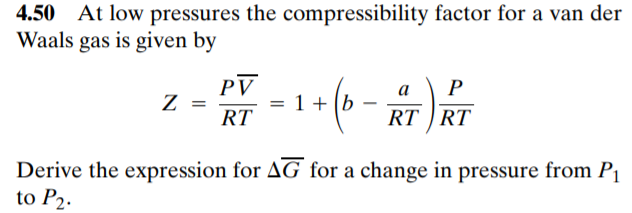
Solved 4.50 At low pressures the compressibility factor for

Van der Waals equation, when pressure correction is ignored, one
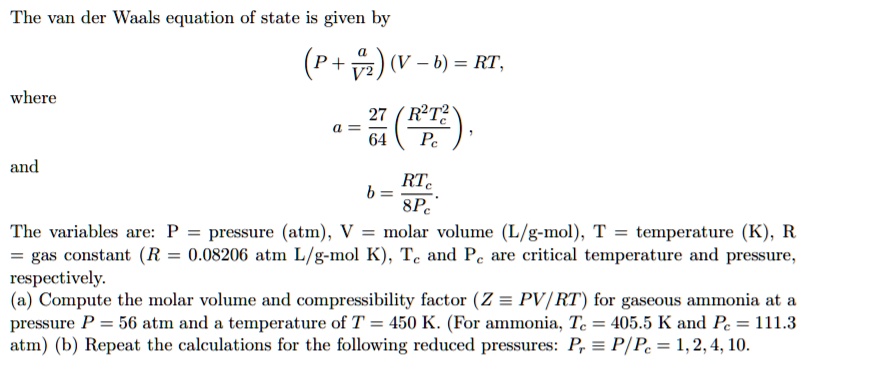
SOLVED: Please help me to solve this problem in Matlab. The van







