If Z is a compressibility factor, van der Waals equation at low
4.8 (744) · $ 10.50 · In stock

Solution For If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 1: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 2: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 3: If Z is a compressibility factor, van der Waals equation at low pressure can be written as
Video solution 4: If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Van Der Waals Equation - an overview
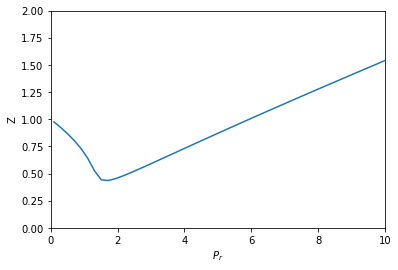
Compressibility factor variation from the van der Waals equation by three different approaches
The given graph represent the variations of compressibility factor (z) = pV/nRT versus p, - Sarthaks eConnect
If Z is a compressibility factor, van der Waals equation at low

If Z is a compressibility factor, van der Waals' equation at low

Multiple Choice Questions on Gas Laws and Kinetic Theory

image.slidesharecdn.com/unit10realgasesvdwfl14fina
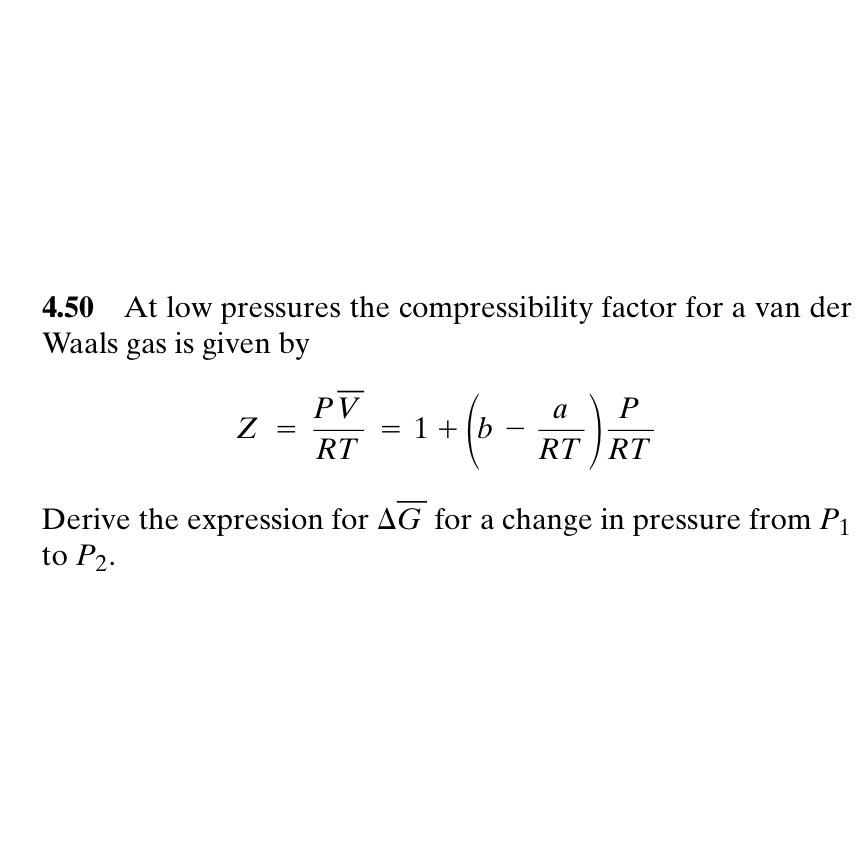
Solved 4.50 At low pressures the compressibility factor for

If Z is a compressibility factor, van der Waals equation at low
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as

Real gases 1.4 Molecular interactions 1.5 The van de Waals equation 1.6 The principle of corresponding states Real gases do not obey the perfect gas law. - ppt download






