a) A gas at 250 K and 15 atm has a molar volume 12 per cent
4.7 (274) · $ 18.99 · In stock

Chapter_132-27-2011-90257PM

Exam questions.pdf - Focus 1 E1A.1 a Express i 108 kPa in torr and

Answered: T = 23 The expression you should be…
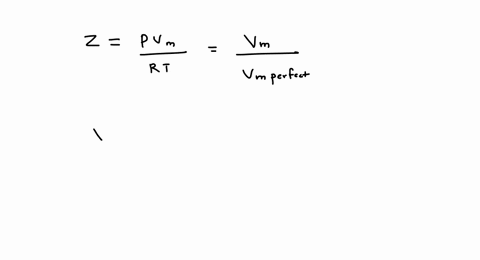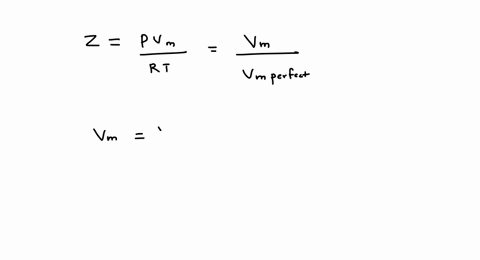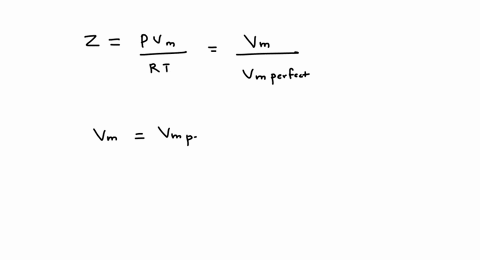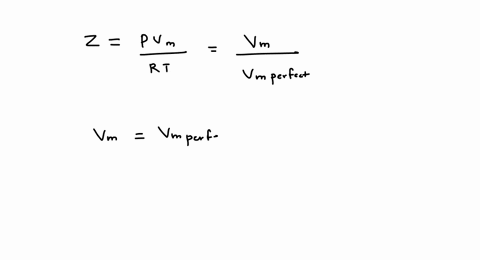
SOLVED: A gas at 250 K and 15 atm has a molar volume 12 percent smaller than that calculated from the perfect gas law. a) Calculate the compression factor under these conditions.

Solved Test Prep Questions A constant-volume perfect gas

A gas mixture at 350 K and 300 kPa has the following volumet

An ideal gas at a pressure of 152 kPa is contained in a bulb of u

PDF) 38 1 THE PROPERTIES OF GASES Discussion questions

59-240 Physical Chemistry - Question Set #4 - Lecture 4 - v. 1.1

The phase diagram for SO2 is shown here. (e) At which of the thre

Sat chemistry notes by Andora Conti - Issuu
A sample of a gas weighs 1.25g at 28 C° occupying volume of 2.50

Answered: 1. A 0.5 mole of gas at temperature 300…
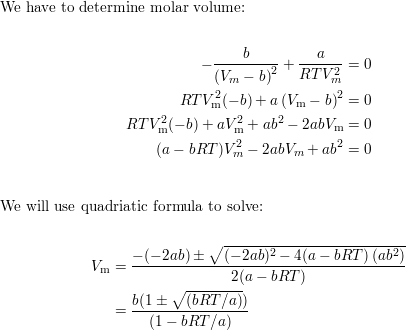
Is there a set of conditions at which the compression factor







