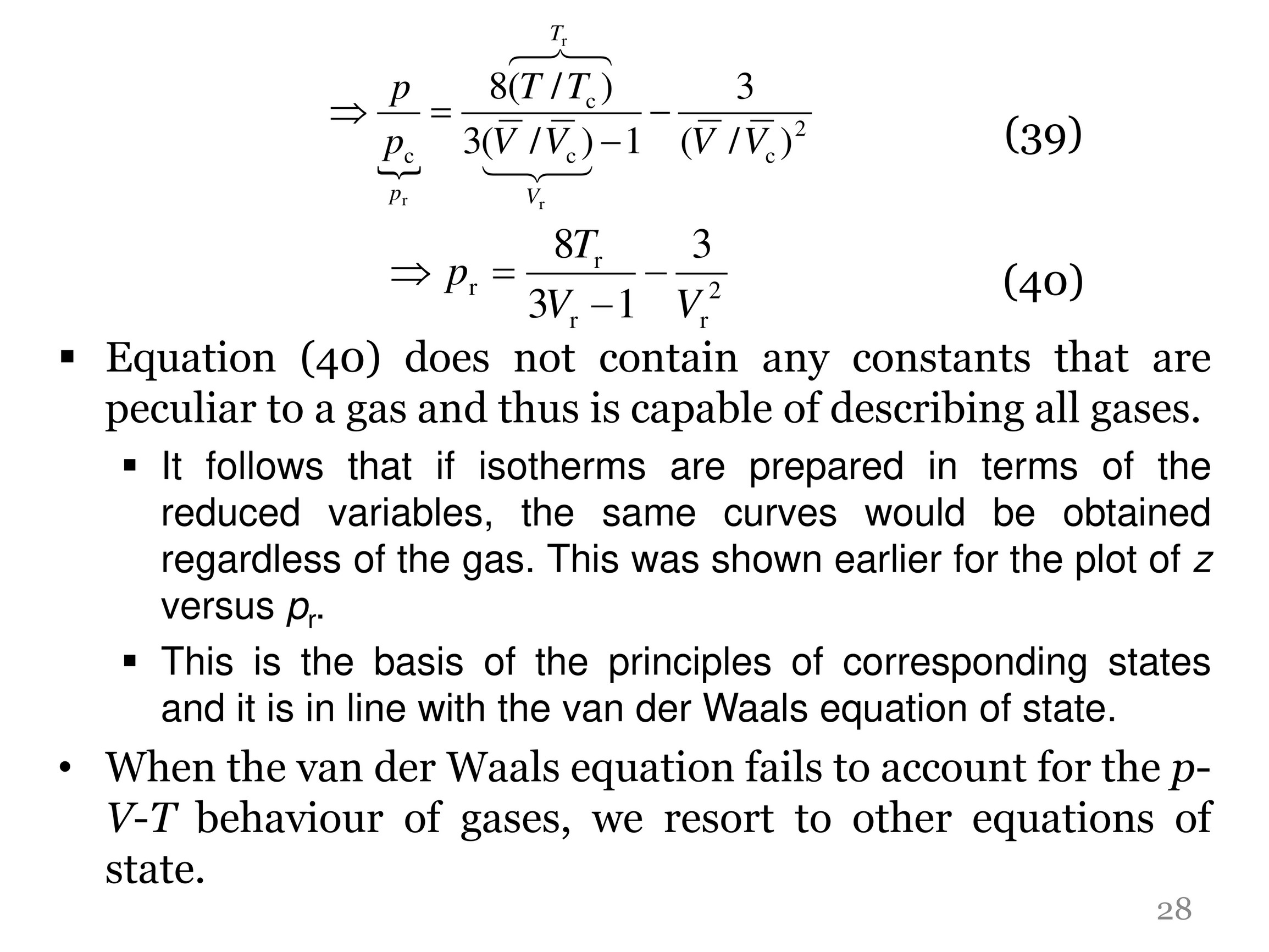
The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6
4.8 (552) · $ 32.99 · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:the compressiblity factor for a gas obeying vander waals equation of state is given byvvbrtv2
Click here👆to get an answer to your question ✍️ The compressiblity factor a gas obeying van der Waals- equation of state is given by V V-b RTV -2- a - RTV V-b V-b RTV -3- Va -4- RTV V-6

The compressibility factor of a van der Waals gas the critical point is equal to
Solved Deduce The Correct Expression For Calculating VR, 52% OFF

vocab.txt · sentence-transformers/allenai-specter at main
Solved Deduce The Correct Expression For Calculating VR, 52% OFF

The compressiblity factor a gas obeying van der Waals' equation of state is given by V V-b RTV (2) a ✓ RTV V-b V-b RTV (3) Va (4) RTV V-6

At a high pressure, the compressibility factor (Z) of a real gas is us

The compressiblity factor a gas obeying van der Waals' equation of state is given by V-b RTV RTV V-b V-b RTV RTV V-b

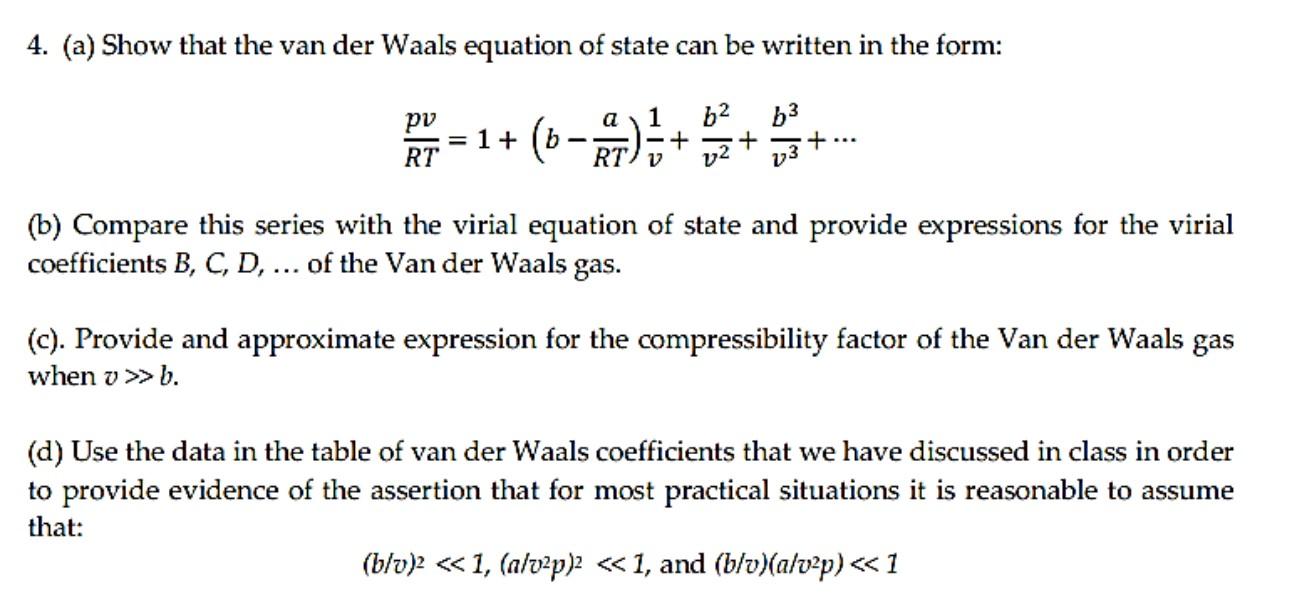
The virial form of van der Waal's gas equation is PV=RTleft(1+dfrac{B}{V }+dfrac{C}{V^2}+.right)=RT(1+B'P+C'P^2+.). The second virial coefficient or argon gas 262.5K is -1 l mol^{-1}. What is the density of argon gas 262.5K and
The value of compression factor at the critical state of a vander waals gas is
Solved 4. (a) Show that the van der Waals equation of state







