Health-related quality of life and quality-adjusted progression
4.9 (712) · $ 21.99 · In stock
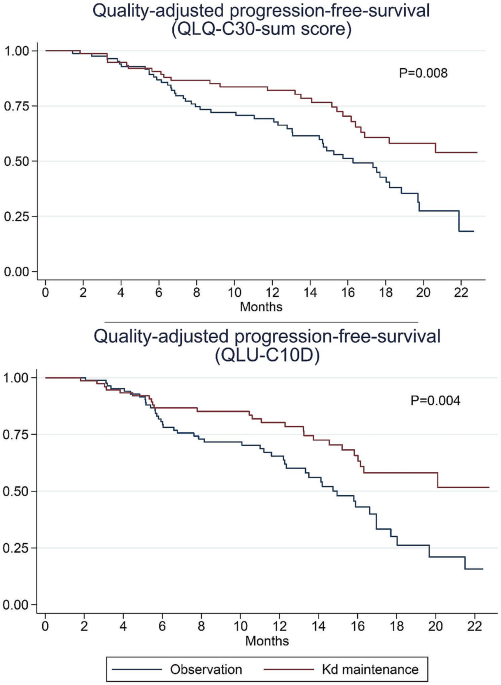
Background Decisions regarding maintenance therapy in patients with multiple myeloma should be based on both treatment efficacy and health-related quality of life (HRQL) consequences. In the CARFI trial, patients with first relapse of multiple myeloma underwent salvage autologous stem cell transplantation (salvage ASCT) before randomization to carfilzomib-dexamethasone maintenance therapy (Kd) or observation. The primary clinical endpoint was time to progression, which was extended by 8 months by Kd. The aim of this paper is to present the all HRQL endpoints of the CARFI trial including the HRQL effect of Kd maintenance therapy relative to observation. The primary HRQL endpoint was assessed by EORTC QLQ-C30 Summary score (QLQ-C30-sum) at 8 months follow-up. A key secondary HRQL endpoint was quality-adjusted progression-free-survival (QAPFS). Methods HRQL was assessed with EORTC QLQ-C30, EORTC QLQ-MY20 and FACT/GOG-Ntx at randomization and every second month during follow-up. HRQL data were analyzed with linear mixed effect models until 8 months follow-up. QAPFS per individual was calculated by multiplying progression-free survival (PFS) by two quality-adjustment metrics, the QLQ-C30-sum and EORTC Quality of Life Utility Measure-Core 10 dimensions (QLU-C10D). The QAPFS per treatment group was estimated with the Kaplan-Meier method. P < 0.05 was used for statistical significance, and a between-group minimal important difference of 10 points was interpreted as clinically relevant for the QLQ-C30-sum. Results 168 patients were randomized. HRQL questionnaire compliance was 93%. For the QLQ-C30-sum, the difference of 4.62 points (95% confidence interval (CI) -8.9: -0.4, p = 0.032) was not clinically relevant. PFS was 19.3 months for the Kd maintenance group and 16.8 months for the observation group; difference = 2.5 months (95% CI 0.5; 4.5). QAPFS based on the QLQ-C30-sum for the Kd maintenance group was 18.0 months (95% CI 16.4; 19.6) and for the observation group 15.0 months (95% CI 13.5; 16.5); difference = 3.0 months (95% CI 0.8–5.3). QAPFS based on the QLU-C10D for the Kd maintenance group was 17.5 months (95% CI 15.9; 19.2) and 14.0 months (95% CI 12.4; 15.5) for the observation group; difference = 3.5 months (95% CI 1.1–5.9). Conclusions Kd maintenance therapy after salvage ASCT did not adversely affect overall HRQL, but adjustment for HRQL reduced the PFS compared to unadjusted PFS. PFS of maintenance therapy should be quality-adjusted to balance the benefits and HRQL impact.

Progress towards the Sustainable Development Goals
Salvage autologous transplant and lenalidomide maintenance vs. lenalidomide/dexamethasone for relapsed multiple myeloma: the randomized GMMG phase III trial ReLApsE

The sequential steps for estimating quality-adjusted life years (QALYs).
Measuring Quality of Life in Ovarian Cancer Clinical Trials--Can We Improve Objectivity and Cross Trial Comparisons? - Document - Gale OneFile: Health and Medicine

JPatientRepOutcomes (@JPRO_ISOQOL) / X
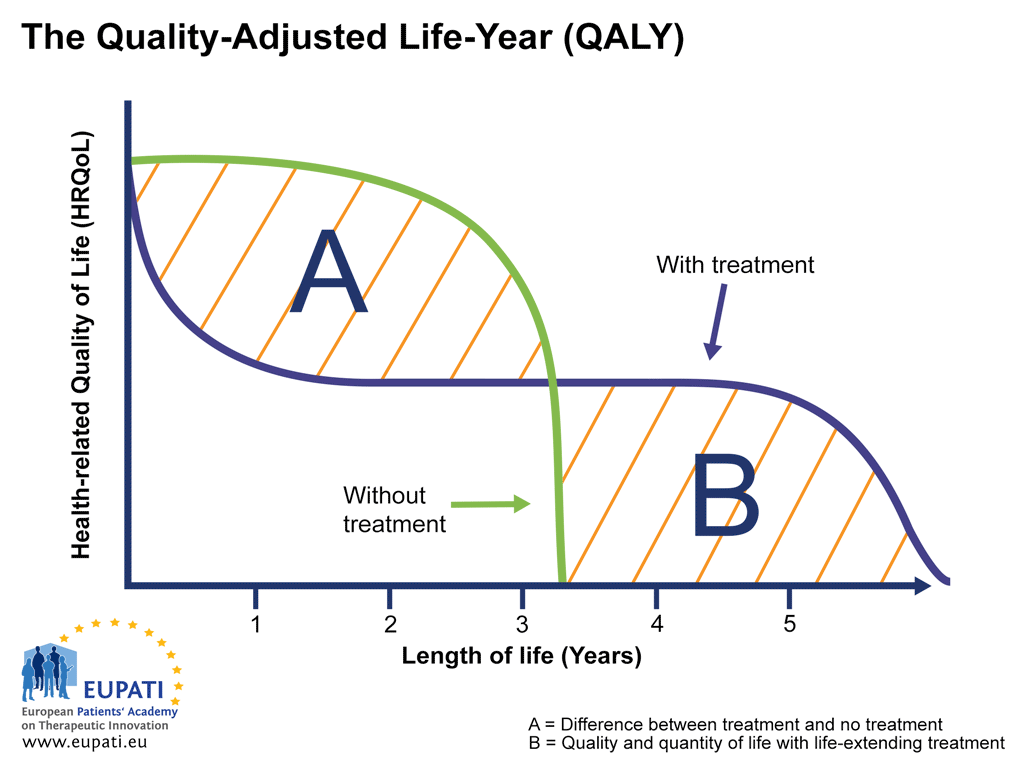
Measuring Health-related quality of life (HRQoL) - EUPATI Toolbox

PDF) Nutrition economics - Characterising the economic and health impact of nutrition
PDF) Estimation of minimally important differences and responder definitions for EORTC QLQ‐MY20 scores in multiple myeloma patients
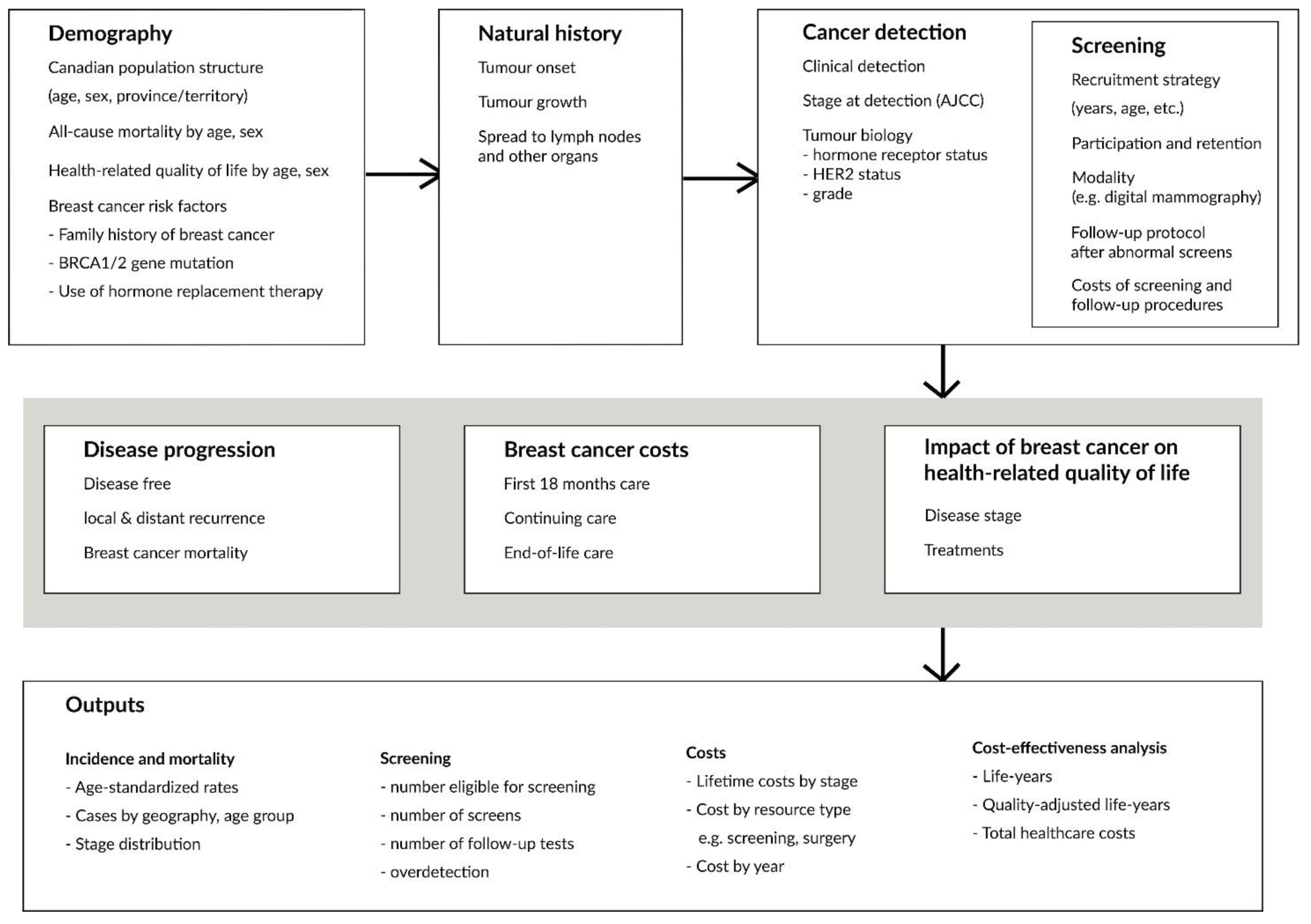
Current Oncology, Free Full-Text

Health-related quality of life and quality-adjusted progression free survival for carfilzomib and dexamethasone maintenance following salvage autologous stem-cell transplantation in patients with multiple myeloma: a randomized phase 2 trial by the Nordic

Health-related quality of life of carfilzomib- and daratumumab-based therapies in patients with relapsed/refractory multiple myeloma, based on German benefit assessment data

Higher order models for the EORTC QLQ-C30 (models 1e7 was taken from
:max_bytes(150000):strip_icc()/TermDefinitions_PercapitaGDP-09e9332fe3d04e68b34e676554168077.jpg)
GDP Per Capita: Definition, Uses, and Highest Per Country
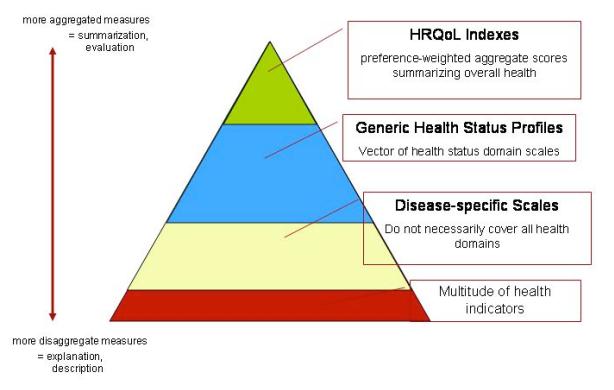
Measuring health related quality of life

PDF) Mapping SF-36 onto the EQ-5D index: How reliable is the relationship?