Solved What does the mean free path of a molecule in a gas
4.8 (712) · $ 23.50 · In stock
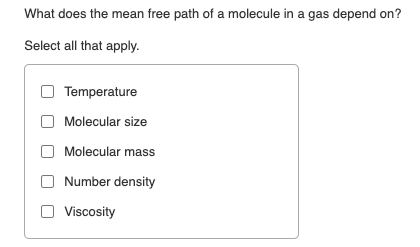

The mean path of molecules of a gas (radius 'r') is inversely proportional tor^3r^2rsqrt r
Calculate the mean free path of a gas molecule with diameter 4 Å if the pressure of the gas is 1.013 × 10^5 N/m^2 - Sarthaks eConnect
assertion . mean free path of gas molecule varies inversely as density of the gas reason. mean free path varies inversely as pressure of the gas ?? what is correct explaination of assertio
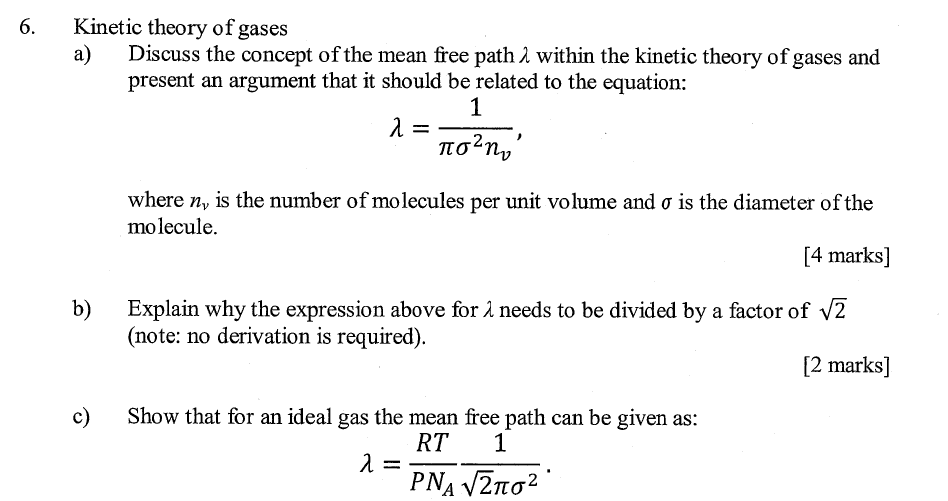
Solved 6. Kinetic theory of gases Discuss the concept of the
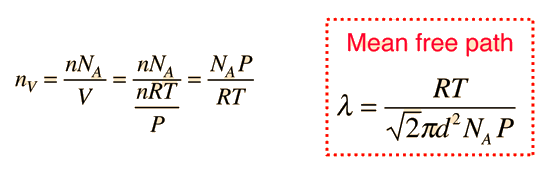
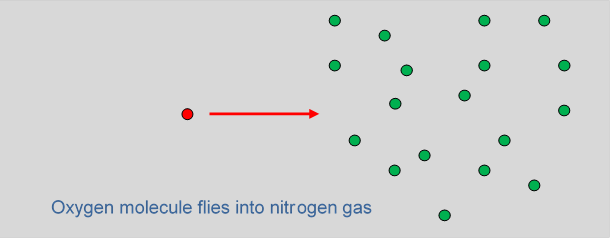
Mean Free Path, Molecular Collisions
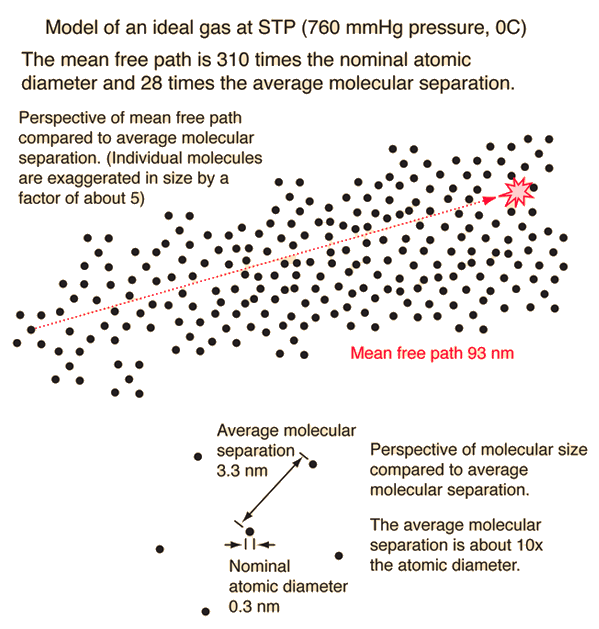
Mean Free Path, Molecular Collisions
Boundary Conditions
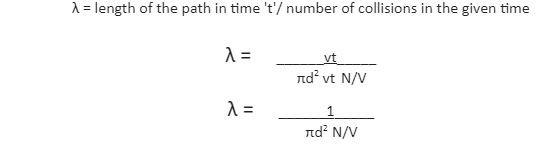
Essential Study Notes on Mean Free Path

The mean free path for a gas, with molecular diameter d and number density n can be expressed as :
![Telugu] (A) is true but (R ) is false](https://static.doubtnut.com/ss/web/7182309.webp)
Telugu] (A) is true but (R ) is false
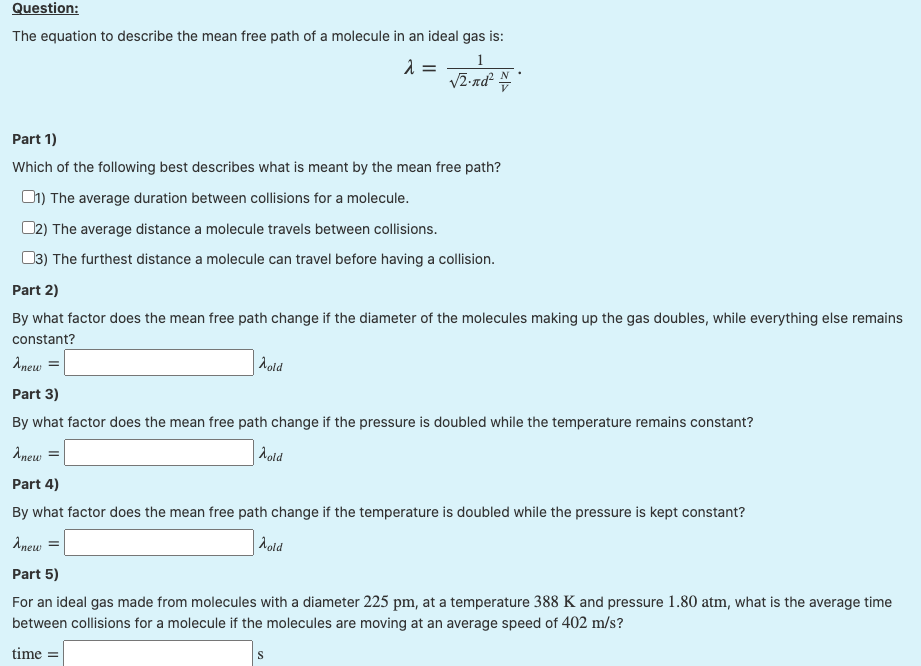
Solved Question: The equation to describe the mean free path
Derive the expression for mean free path of the gas. - Sarthaks eConnect
The diameter of a gas molecules is 2.4 x 10^-10m. Calculate the mean free path at N.T.P. Given - Sarthaks eConnect

Mean Free Path Example
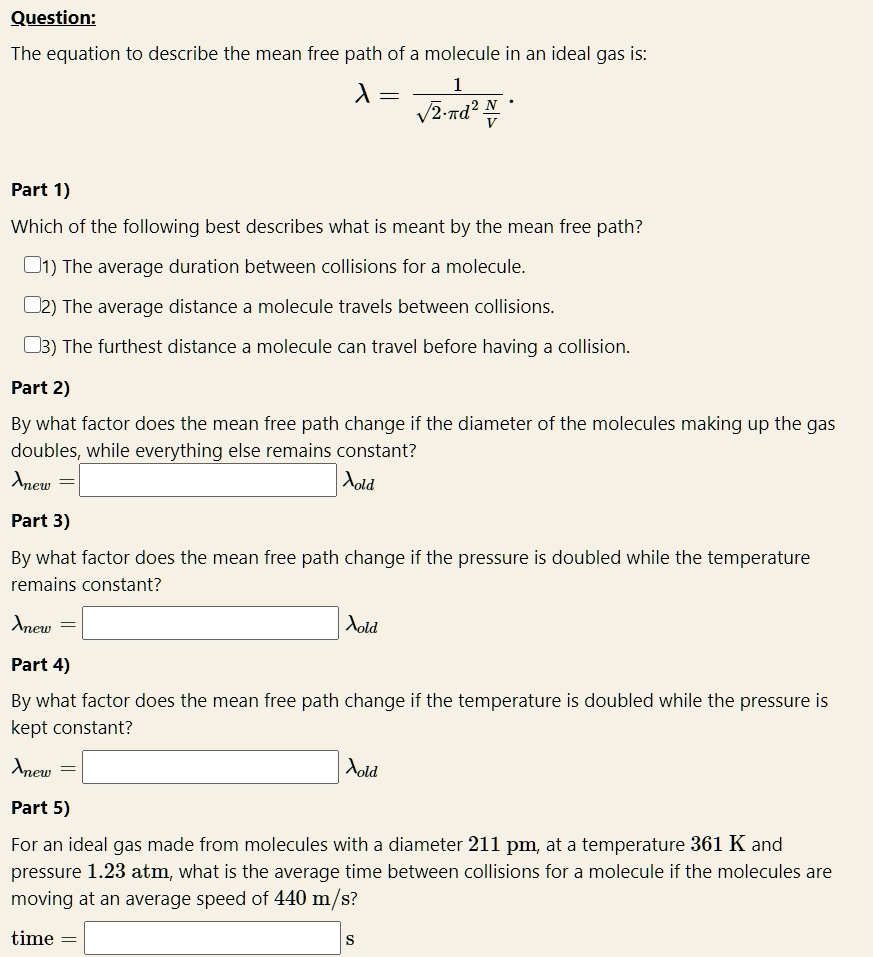
SOLVED: Question: The equation to describe the mean free path of a molecule in an ideal gas is: λ = Vzrdν Part 1) Which of the following best describes what is meant