The value of compression factor at the critical state of a vander waals gas is
4.8 (413) · $ 29.00 · In stock
The value of compression factor at the critical state of a vander waals gas is

Van der Waals Equation - Derivation, Relation Between Ideal Gas Law, Application
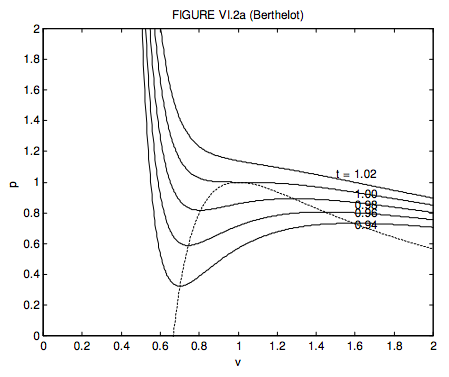
6.3: Van der Waals and Other Gases - Physics LibreTexts

Answered: Compression factor of a gas with van…

The compressibility factor of H(2)(g) at its critical condition if it

108. Which of following statement (s) is true 1 - Slope of isotherm critical point is maximum. 103. 11 - Larger is the value of T, easier is the liquification of gas.X
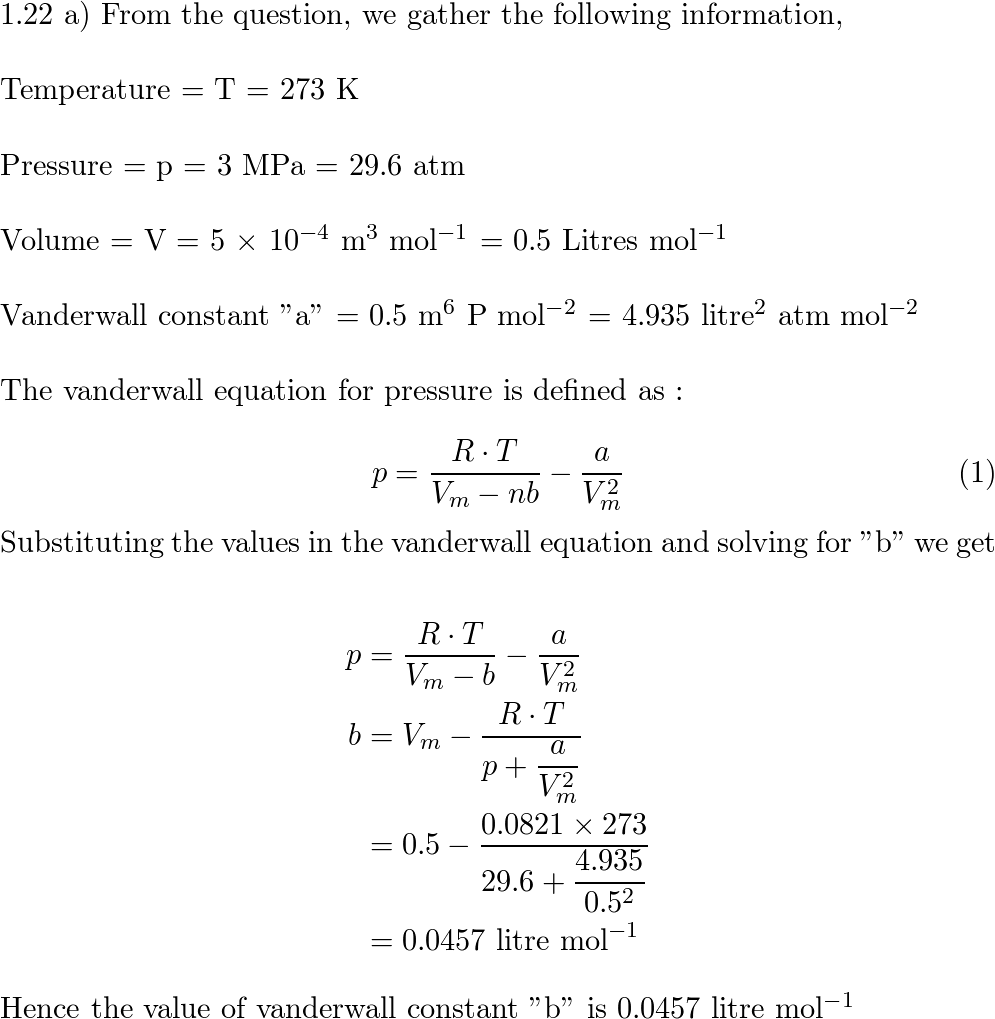
a) A certain gas obeys the van der Waals equation with $a =
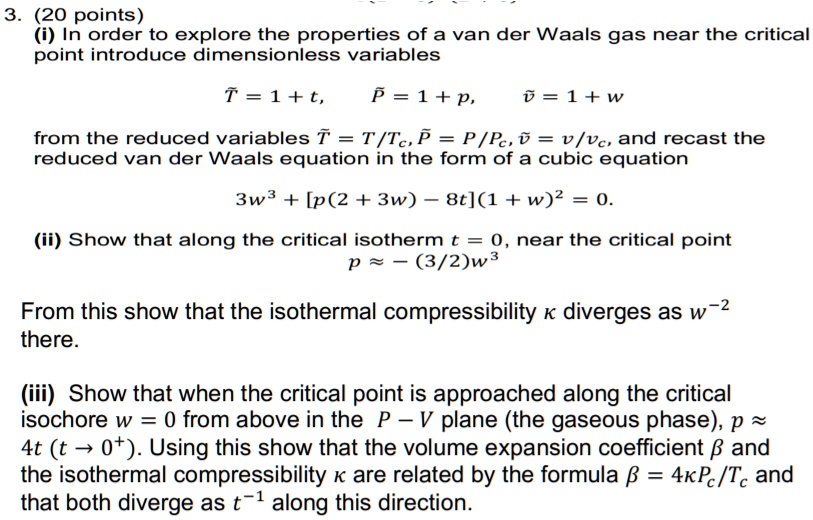
SOLVED: (i) In order to explore the properties of a van der Waals gas near the critical point, introduce dimensionless variables: T = 1 + t, P = 1 + p, V =

At a high pressure, the compressibility factor (Z) of a real gas is us
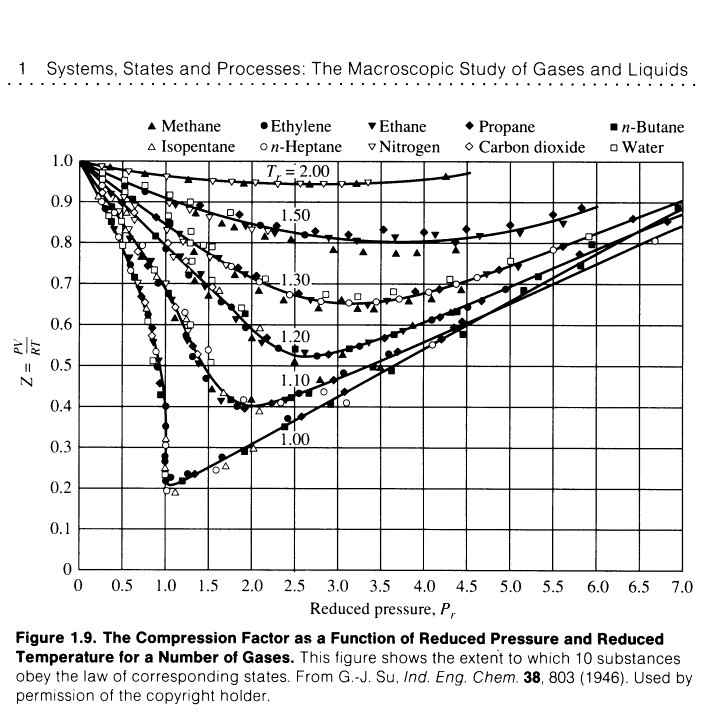
Chapter 1 Properties of Gases

What is the value of z (compressibility factor) for a vander waal gas at critical







