117. Compressibility factor H, behaving as rea gas is 1) 1 RTV 3) 1+- RT 4) (1-a) 18. If V is the observed molor unlum
4.6 (447) · $ 26.50 · In stock

Click here:point_up_2:to get an answer to your question :writing_hand:117 compressibility factor for h behaving as reagas is1 1rtv31rt41a18 if v is the observed
Click here👆to get an answer to your question ✍️ 117- Compressibility factor H- behaving as rea gas is 1- 1 RTV 3- 1- RT 4- -1-a- 18- If V is the observed molor unlum

The compressibility factor Z a low-pressure range of all gases except hydrogen is:Z=(1+ displaystylefrac{a}{V_{m}RT})Z=(1-displaystylefrac{a}{V _{m}RT})Z=(1+displaystylefrac{Pb}{RT})Z = ( 1 - displaystylefrac{Pb}{RT})

2012good-Book-An Introduction To Acoustics, PDF, Fluid Dynamics

The compression factor (compressibility factor) for `1 mol` of a van der Waals gas at

Chemosensors, Free Full-Text

Physics Textbook, PDF, Torque

An unknown gas at 37.1 ∘c and 1.00 atm has a molar mass of 30.07 g/mol. assuming ideal behavior, what is

Gasdynamics PDF, PDF, Mach Number

Compressibility factor for H(2) behaving as real gas is
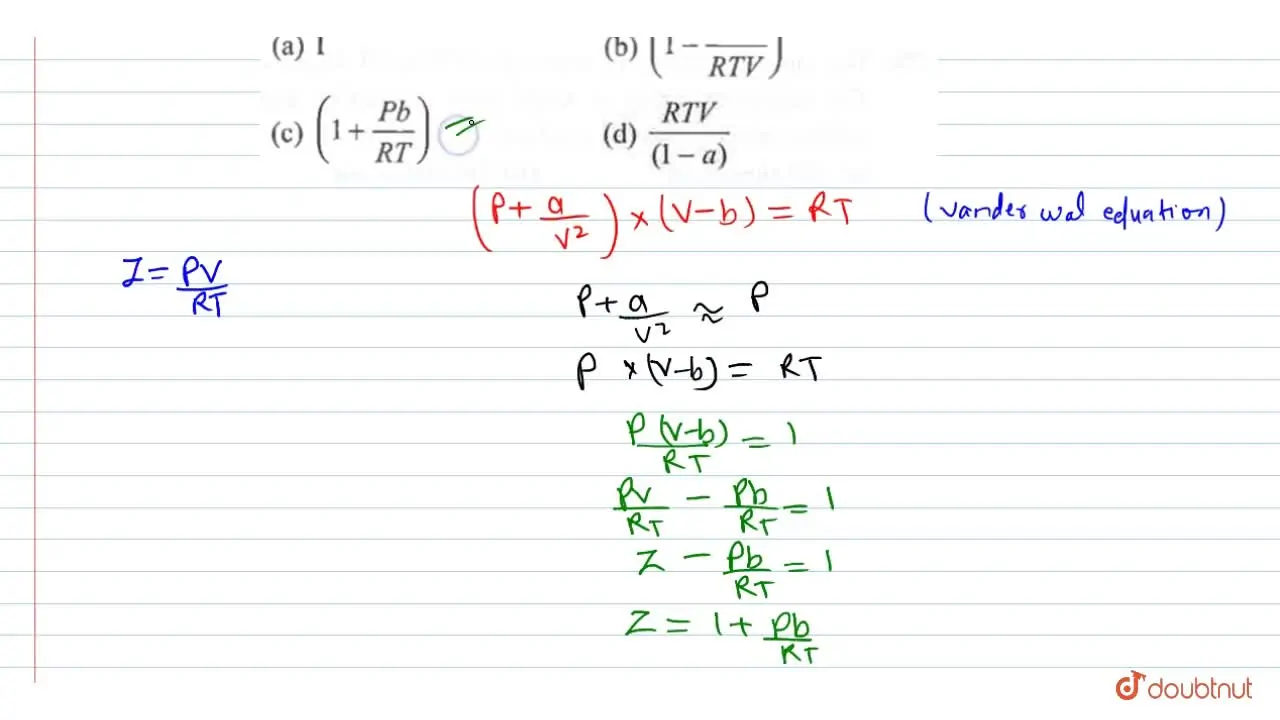
Compressibility factor for H(2) behaving as real gas is

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

Non-Ideal Gas Behavior Chemistry: Atoms First
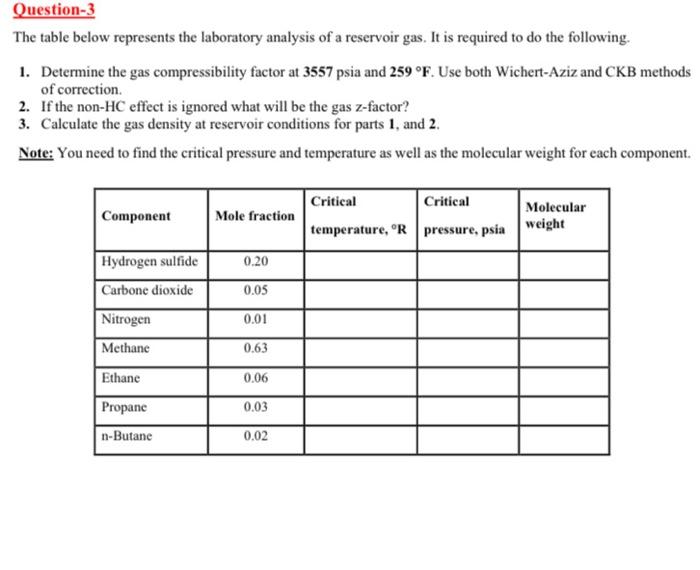
Solved The table below represents the laboratory analysis of

1 - Argonne National Laboratory
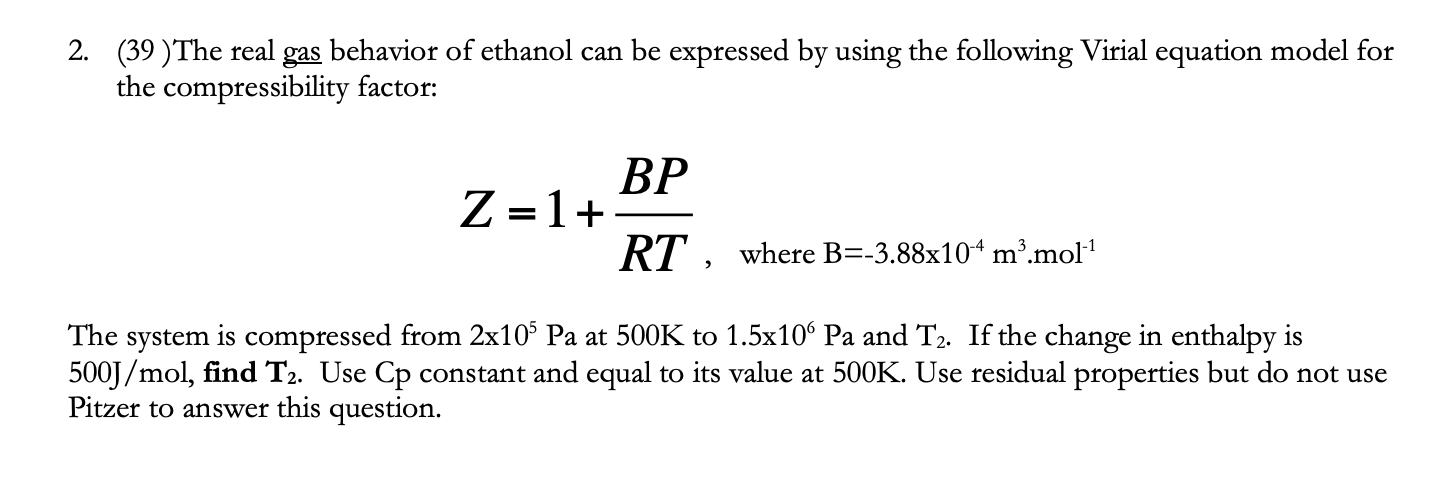
Solved The real gas behavior of ethanol can be expressed by







