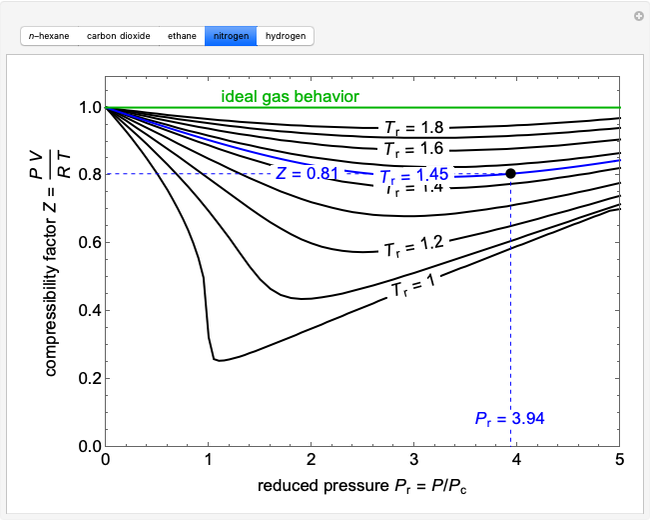
The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n
4.5 (354) · $ 23.99 · In stock
![The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n](https://www.vedantu.com/question-sets/a60a955d-f16d-432e-852e-8dcb41060ecf8392656688535846153.png)
The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n A.For the gas A, a=0 and its dependence

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

For the gas C which is a typical real gas for which neither a nor b =0
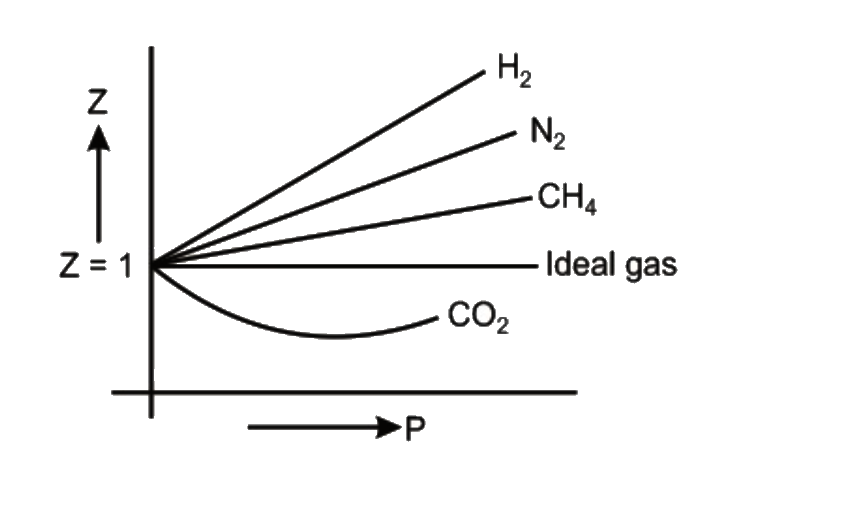
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with

The given graph represents the variation of compressibility factor Z vs P for three gases A, B and C.Identify the incorrect statements.
![Telugu] The variation of compressibility factor (Z) with pressure (p](https://d10lpgp6xz60nq.cloudfront.net/physics_images/MTG_NEET_GID_CHE_XI_C05_E01_187_Q01.png)
Telugu] The variation of compressibility factor (Z) with pressure (p

variations of 2 12.7 (a) eb (c)-(ar (d) - 6. The given graph represent the variations (compressibility factor (Z)=- gases A, B and C. Identify the only incorrect statement pl) versus p

The given graph represents the variation of Z(compressibility factor =- PV nRT ) versus P, three real gases A, B and C. Identify the only incorrect statement. Ideal gas P (atm) (A)

Compressibility factor, Z of a gas is given as Z = pV / nRTi What is the value of Z for an ideal gas?ii For real gas what will be the effect