At certain states, the p-v-T data of a gas can be expressed
5 (517) · $ 11.99 · In stock
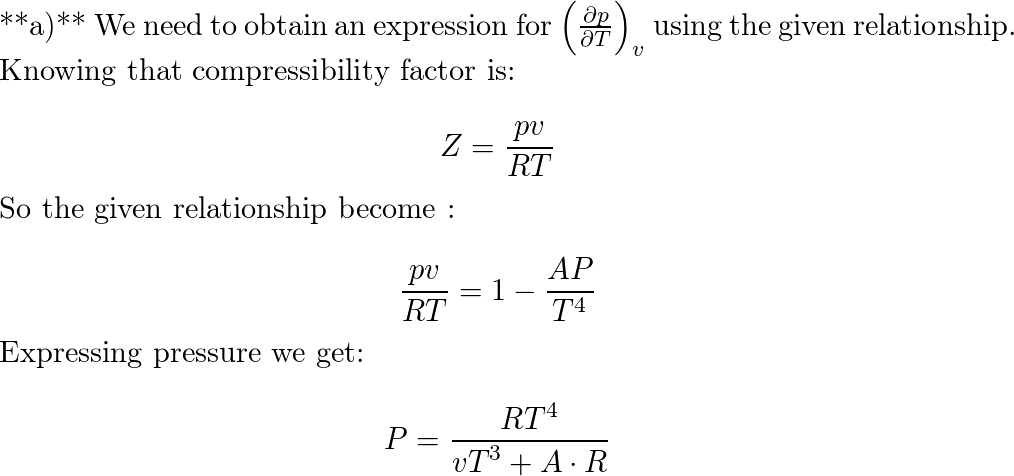
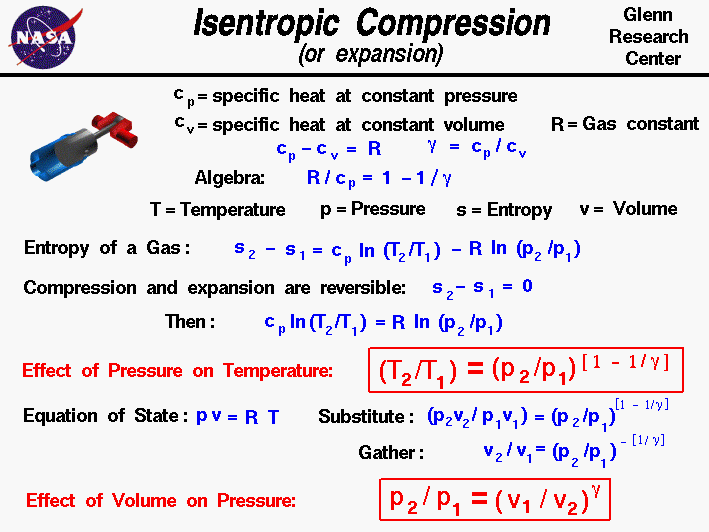
Isentropic Compression or Expansion

Gas Laws M. L. Watson. An ideal gas is defined as one in which all collisions between atoms or molecules are perfectly elastic and in which there are. - ppt download

SOLVED: Consider a monoatomic, imperfect gas, whose fugacity is expressed as a Taylor series expansion of the pressure: fT,P = P1 + zTP + zTp + , with temperature-dependent coefficients zx(T). a)

SOLVED: For an adiabatic, reversible expansion or compression, derive an exact expression that relates the initial and final temperatures of an ideal gas, Ti and T2, to the initial and final volumes
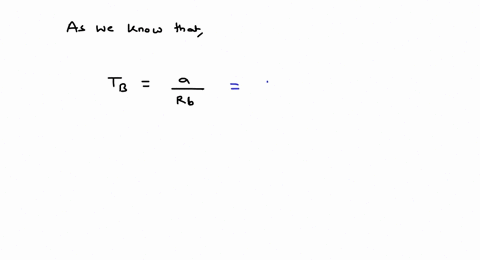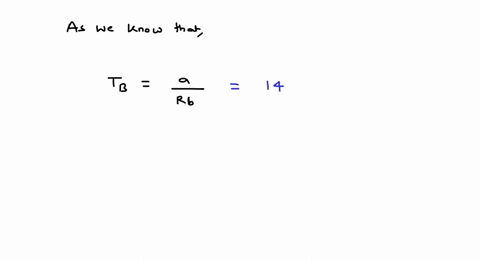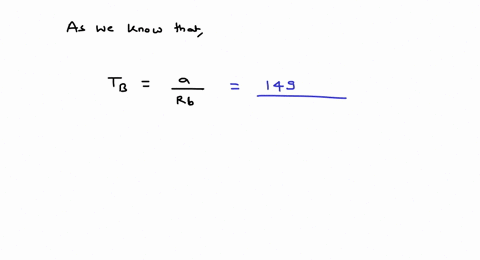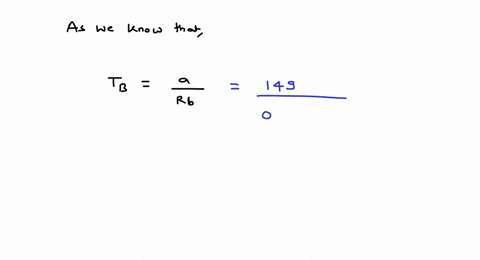
⏩SOLVED:For values of z near 1, it is a good approximation to write…
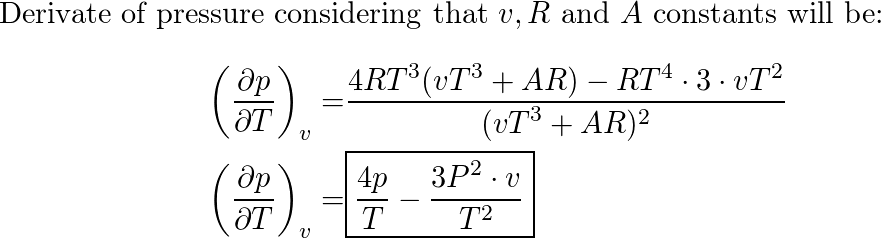
d2nchlq0f2u6vy.cloudfront.net/21/07/31/f27498dd347

The Red Ball Express

⏩SOLVED:For values of z near 1, it is a good approximation to write…

⏩SOLVED:Using Amagat's law, show that Zm=∑i=1^k yi Zi for a real-gas…
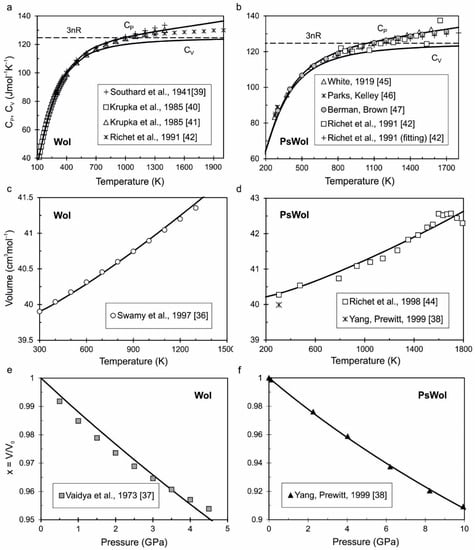
Minerals, Free Full-Text
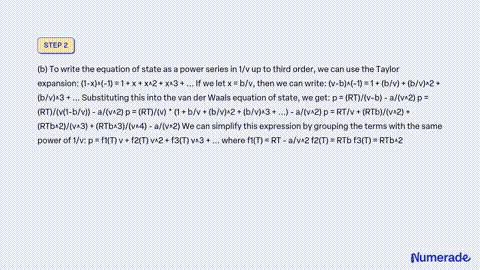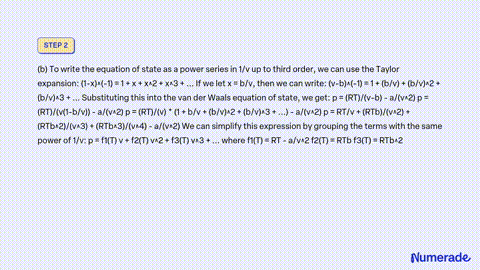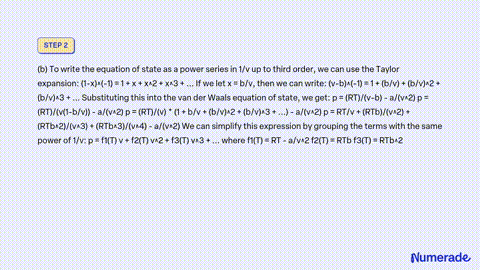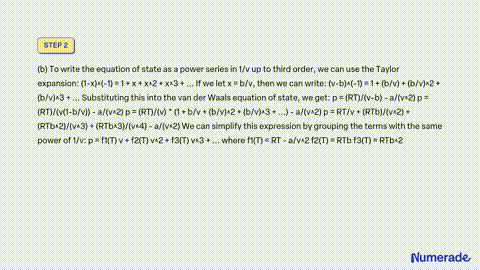
SOLVED: Consider a monoatomic, imperfect gas, whose fugacity is expressed as a Taylor series expansion of the pressure: fT,P = P1 + zTP + zTp + , with temperature-dependent coefficients zx(T). a)

⏩SOLVED:The temperature of an ideal gas having constant specific…

⏩SOLVED:At certain states, the p-v-T data for a particular gas can…

Compressibility factor for methane.
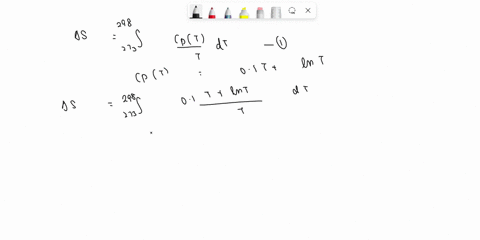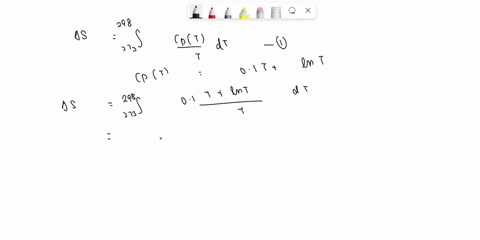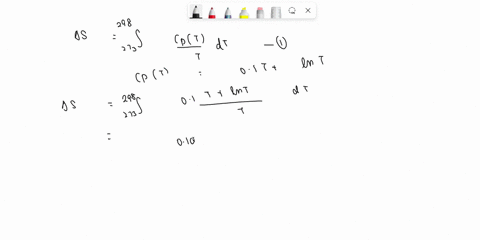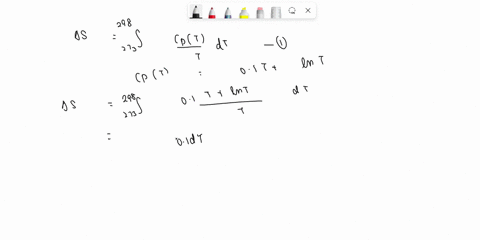
⏩SOLVED:Develop an expression for the variation in temperature with…






